By Brad Buecker, ChemTreat and Ken Kuruc, Hach
With the decline of coal-fired power generation and the upswing in renewables, a large bridge between the two has been and continues to be simple- and especially combined cycle power generation with natural gas being the primary fuel.
Very common for existing and planned combined cycle power plants is operation with minimal staff. For the gas turbine portion of these plants, “lean and mean” operation may be satisfactory. But often overlooked is that the heat recovery steam generators (HRSGs) require significant attention to prevent corrosion and deposition in these units, which otherwise could impact unit availability and potentially even threaten employee safety in some cases. This article focuses upon the critical on-line water/steam chemistry analyses that are necessary for plant personnel to optimize HRSG performance and reliability.
Sampling Points and Monitoring Parameters
The samples of primary importance throughout the steam-generating network are:
- Makeup treatment system
- Condensate pump discharge
- Feedwater or economizer inlet
- Boiler water
- Saturated steam
- Main and reheat steam
Makeup Treatment System
Even in the tightest steam generators a small amount of process water/steam continually escapes. These losses must be made up with high-purity water. Most common as the core process of makeup systems is reverse osmosis (RO) followed by either mixed-bed ion exchange (MBIX) or electrodeionization (EDI) to “polish” the RO effluent. RO units typically include a number of instruments to monitor system performance, including pressure, temperature, flow, and specific conductivity, which are the subject for a separate discussion. The list below outlines the recommended upper limit for the three recommended sampling parameters of the makeup system effluent.
- Specific conductivity (S.C.): ≤0.1 µS/cm
- Silica: ≤10 parts per billion (ppb)
- Sodium: ≤2 ppb
These measurements ensure that high-purity water is being distributed to the steam generators. A rise in any of the values indicates that either the MBIX resin has reached exhaustion or that a problem has occurred in the EDI unit. Prompt corrective action is necessary.
(Note: In this and the following sections, the normal upper limit, or range, for each parameter is included. This data and many other details may be found in published documents by the Electric Power Research Institute [EPRI]. However, these documents are usually only available to EPRI members. The International Association for the Properties of Water and Steam [IAPWS] offers technical documents that have similar, albeit more condensed, information, which are downloadable from their website, www.iapws.org.)
Condensate Pump Discharge (CPD)
In steam generating power units, the primary location for potential contaminant ingress is the condenser, and particularly water-cooled condensers where a tube leak(s) allows cooling water to infiltrate the high-purity condensate. Cooling water in-leakage will introduce a variety of impurities to the steam generator, which, when subjected to the harsh environment in the boilers (the common term for HRSGs is evaporators) can cause serious problems.
Recommended continuous CPD analyses are:
- Cation Conductivity (CACE): ≤0.2 µS/cm
- S.C.: Consistent with pH
- Sodium: ≤2 ppb
- Dissolved Oxygen: ≤20 ppb
- pH: 9.6 to 10.0 (This is the pH range for the most common HRSG design, the triple-pressure, feed-forward low-pressure type. The range may be a bit different for other HRSG designs.)
Sodium monitoring is very effective for detecting condenser tube leaks. With a tight condenser, sodium levels in the condensate are normally very low (<2 ppb), and in many cases less than 1 ppb. A rise in sodium provides the earliest indication of a condenser tube leak.
Cation conductivity has been re-designated by some research organizations as “conductivity after cation exchange (CACE)” to represent the fact that the sample is routed through a cation exchange column to replace the cations, e.g., ammonium, sodium, calcium, etc. with hydrogen ions. This creates a very dilute acid solution of primarily trace amounts of chloride and sulfate ions, whose conductivity is then measured. As with sodium, a rise in CACE indicates impurity in-leakage. CACE can be influenced by carbon dioxide ingression, often from increased air in-leakage at the condenser. Thus, becoming increasingly popular is degasified CACE, which utilizes either a re-boiler or nitrogen sparging compartment to remove up to 90% or so of the CO2.
Dissolved oxygen analyses are important for monitoring condenser air in-leakage. A sudden increase in dissolved oxygen may indicate a mechanical failure at or near the condenser, which allows excess air to enter the system.
With regard to specific conductivity and pH, ammonia (or sometimes an amine or ammonia/amine blend) is the pH-conditioning agent for condensate/feedwater. However, direct pH measurement of high-purity water can be tricky, and algorithms have been developed to calculate pH based on S.C. and CACE measurements to provide more accurate results. S.C. in high-purity water is directly correlated to the ammonia concentration, and thus S.C. measurements offer better control of ammonia feed than pH.
A parameter not typically monitored continuously, but which can be of some importance is total organic carbon (TOC). For utility steam generators, the recommended TOC limit in the CPD is 100 ppb.
LP Economizer Inlet/Boiler Feed Pump Discharge
The dominant issue with regard to chemistry control in the HRSG feedwater system is minimization of flow-accelerated corrosion, which the authors discussed in a previous Power Engineering article. [1]
The following parameters are recommended for feedwater chemistry:
- CACE: ≤0.2 µS/cm
- S.C: Consistent with pH
- Sodium: ≤2 ppb
- Dissolved Oxygen (range): 5 to 10 ppb (unless the feedwater system contains copper alloys, which are almost never present in HRSG condensate/feedwater systems)
- pH: 9.6 to 10.0 (This is the pH range for the most common HRSG design, the triple-pressure, feed-forward low-pressure type. The range may be a bit different for other HRSG designs.)
- Iron: ≤2 ppb
The discussion for CACE, S.C., pH, and sodium mirrors that for condensate pump discharge. The measurements can provide valuable redundancy in determining whether a potential upset is due to an actual problem or instrument error.
Note the inclusion of iron in this list. Iron monitoring provides a direct measurement of FAC (or hopefully lack thereof) and the corresponding effectiveness of the feedwater chemistry program. Typically, 90 percent or greater of iron corrosion products generated by FAC are particulate in nature. Several methods exist to monitor carbon steel corrosion, and include:
- Continuous particulate monitoring
- Corrosion product sampling
- Grab sample analysis
With respect to the latter, improved grab sampling techniques are available, in which, with proper sample treatment, iron measurements down to 1 ppb are possible. This method can provide near real-time data of corrosion rates, although on a snapshot basis.


Fig. 1. Iron digestion unit/spectrophotometer for grab samples. Photos courtesy of Hach.
A combination of a simple colorimetric total iron laboratory analysis with a sensitive laser nephelometric analyzer can also provide a method for cost effective, quantitative, real-time corrosion monitoring.
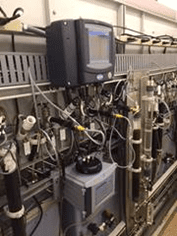
Fig. 2. A nephelometer mounted on a water/steam sample panel. Photo courtesy of Hach.
When properly calibrated, the nephelometric measurement units provided by the instrument can be correlated to total iron concentration values. The iron concentration of the feedwater is a direct indicator of steel corrosion. However, any of several species may be present depending upon the feedwater chemistry employed in the process. These include Fe3O4 (magnetite, gray-black color), α-iron (III) oxide (hematite, red color), and a usually minor concentration of dissolved iron. Each of these species produces a different nephelometric response to visible light. Black magnetite absorbs more and reflects less light than red hematite. Dissolved iron does not produce any nephelometric response. In addition, corrosion products range in size from sub-micron to 10 μm in diameter, with an average diameter of 1 μm. [2] This size range poses another challenge for particle monitoring because nephelometers respond differently to different particle sizes.
These variables make it impossible to create a universal nephelometric calibration for quantification of corrosion products. A calibration which is appropriate for a particular sample location with particular corrosion characteristics will not be accurate for a different application with different parameters. Therefore, quantification of total iron via nephelometry must be accomplished through site-specific calibration.
Evaporator (Boiler) Water
Evaporator water sampling is critical for several reasons. First, poor chemistry control and/or poor monitoring can allow unacceptable carryover of excess impurities to the steam. Secondly, most HRSGs are multi-pressure units, where the chemistry in each circuit is different from the other circuits. Comprehensive monitoring is necessary to ensure proper chemistry throughout the steam generator. Thirdly, the highest heat fluxes occur within the evaporators, and particularly the HP evaporator, of HRSGs. The effects of impurity ingress or poor chemistry are magnified by the high temperatures and pressures in these circuits. Consider the classic issue of hydrogen damage, which has plagued high-pressure units for decades.
In this mechanism, the most serious corrosive agent, chloride, that enters during a cooling leak can concentrate under waterwall tube deposits and generate acid. The following equation outlines a common mechanism:
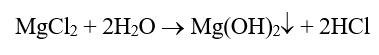
Acid generation is problematic in its own right, but the very small hydrogen atoms will penetrate the steel matrix and then react with carbon in the steel.
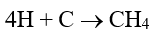
Formation of voluminous methane molecules induces cracking, which can then induce failures with very little metal loss.

Fig. 3. Hydrogen damage. Notice the thick-lipped failure, showing little metal loss.
Author Brad Buecker once directly observed the after-effects of severe hydrogen damage on a 1,250 psig conventional steam generator, where the extensive corrosion required complete replacement of the waterwall tubes. [3] Hydrogen damage remains one of the leading corrosion mechanisms in modern steam generators, and is why, as the list below indicates, immediate unit shutdown is required if the boiler water pH drops below 8.0.
Recommended boiler water analyses include:
- pH (<8.0, immediate boiler shutdown)
- CACE
- Specific Conductivity
- Chloride
- Silica
- Phosphate (for those units on phosphate treatment)
- Iron: <5 ppb
The reader will notice no direct limits for most parameters, with the exception of a “drop-dead” lower limit for pH. This is due to the fact that the limits or control ranges are variable based on boiler pressure. EPRI and IAPWS guidelines provide details on how to calculate the proper ranges for any system, where some adjustments may be necessary based upon operating data.
Comment is necessary regarding phosphate. For decades, tri-sodium phosphate (Na3PO4) has been a core boiler water treatment chemical in many drum units. However, control of the phosphate concentration is difficult due to the compound’s reverse solubility, aka “hideout,” above 300°F. Some plant personnel, especially in the power industry, have switched to a caustic (NaOH) feed to eliminate phosphate hideout, but great care is required with these programs to prevent caustic gouging of waterwall tubes. To avoid such issues, inclusion of a condensate polisher in the unit design offers the opportunity to eliminate phosphate or caustic from the boiler water treatment program.
Steam
Steam purity measurements are extremely important, in large part because the turbine is the most finely-machined and expensive piece of equipment in the entire system. Contaminant deposition on turbine blades can lead to corrosion and possible blade failures, which represent a potentially catastrophic situation with the turbine spinning at several thousand rpm. Core monitoring parameters include the following:
- CACE: ≤0.2 µS/cm
- Sodium: ≤2 ppb
- Silica: ≤10 ppb
Sodium provides a direct indication of salt or sodium hydroxide carryover with the steam. Salts will settle in the last rows of the low-pressure turbine, where they can cause pitting and subsequent stress corrosion cracking (SCC) and corrosion fatigue (CF) of turbine blades and rotors. Sodium hydroxide carryover is a very serious issue, as caustic can quickly induce SCC of turbine components.
CACE provides an indirect measurement of chloride and sulfate carryover, and the ≤0.2 µS/cm value has been a long-time guideline for turbine manufacturers. However, the accuracy of CACE is suspect for chloride and sulfate. Now available is reliable instrumentation to monitor trace levels of these two impurities. [4] Current recommended limits for chloride and sulfate are 2 ppb, but in a well-operated unit they can and should be much lower.
It has long been known that silica in steam will precipitate on turbine blades. While the compound is not corrosive, it can influence turbine aerodynamics and reduce efficiency. Thus, the 10-ppb recommended limit above.
Several steam sampling points are available in power generating units. These include saturated, main, and reheat steam samples. Main and reheat steam are the most important, as they provide data on impurities directly entering the turbine, which can also come from contaminated attemperation water. Analysis of saturated steam is less important on a continual basis, but can be valuable periodically to check for mechanical carryover issues from steam drums, with a common cause being damaged or failed moisture separators in the drums. Sodium monitoring is best for this evaluation.
Disclaimer: This discussion represents good engineering practice developed over many years of research and practical experience. However, it is the responsibility of the plant owners to develop reliable monitoring systems based on consultation with industry experts. Many additional details go into the design and subsequent operation of a water/steam chemistry sampling system.
References
- Buecker, B., Kuruc, K., and L. Johnson, “The Integral Benefits of Iron Monitoring for Steam Generation Chemistry Control”; Power Engineering, January 2019.
- Kuruc, K., and L. Johnson, “New Findings on Monitoring Flow-Accelerated Corrosion”; Proceedings of the 35th Annual Electric Utility Chemistry Workshop, June 2-4, 2015, Champaign, Illinois.
- B. Buecker, “Condenser Chemistry and Performance Monitoring: A Critical Necessity for Reliable Steam Plant Operation”; Proceedings of the 60th Annual International Water Conference, October 18-20, 1999, Pittsburgh, Pennsylvania.
- B. Buecker, “An Advancement in Steam Turbine Chemistry Monitoring”; Power Engineering, March 2018.

About the author: Brad Buecker is Senior Technical Publicist with ChemTreat. He has 35 years of experience in or affiliated with the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company’s La Cygne, Kansas station. He also spent two years as acting water/wastewater supervisor at a chemical plant. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances and advanced inorganic chemistry. He is a member of the American Chemical Society, American Institute of Chemical Engineers, American Society of Mechanical Engineers, Association of Iron and Steel Technology, Cooling Technology Institute (via corporate membership), National Association of Corrosion Engineers, the Electric Utility Chemistry Workshop planning committee, the EPRI-sponsored Power Plant & Environmental Chemistry Committee, and the Power-Gen International planning committee. Buecker has authored many articles and three books on power plant and water/steam chemistry topics. He may be reached at bradley.buecker@chemtreat.com.

Ken Kuruc is Industry Account Manager for Fossil Power with Hach. He has 25 years of experience in working with the power industry, primarily surrounding the steam cycle. His focus in early years has been with dissolved gases for corrosion monitoring as part of Orbisphere, which has since been integrated into Hach. Kuruc has a B.S. in Chemistry from John Carroll University (University Heights, OH) and has presented on this subject along with others at power conferences across the U.S. He may be reached at kkuruc@hach.com.
This article was originally published in Power Engineering magazine and has been republished with permission. Click here to read it on the Power Engineering website.
To read this article in Spanish, click here.
To read this article in Portuguese, click here.

