-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
Cooling towers are an essential component of most industrial and commercial facilities. They cool water used for a variety of processes and applications. In a future post, we will be discussing the role cooling towers play in your facility’s sustainability efforts, but today we want to answer the question: how do cooling towers work? Let’s start with the basics.
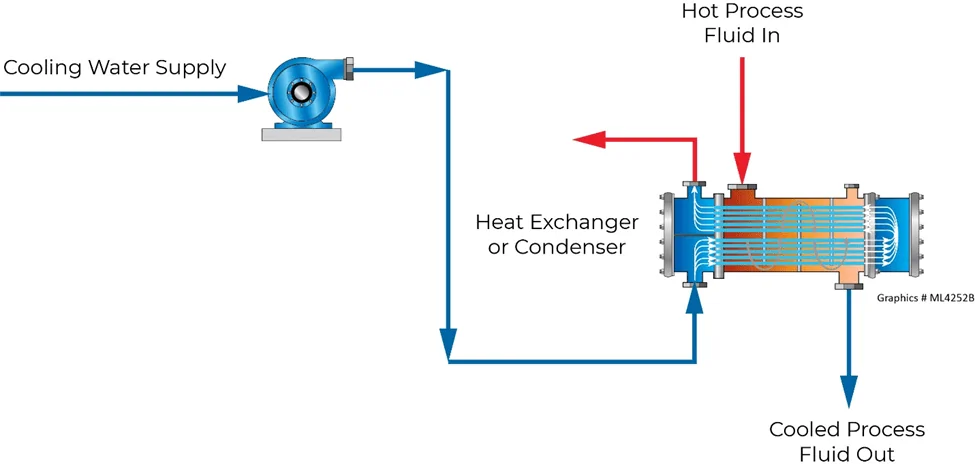
Cooling towers are specialized heat exchangers that are designed to remove waste heat by creating steam. Their function is to pull heat from the water and return cold water to cool down industrial equipment. In a cooling tower, heat is transferred via sensible heat and latent heat.
With cooling towers, most of the heat is transferred to the atmosphere through recirculating cooling water evaporation. Evaporative cooling is frequently used to remove large quantities of heat from processes, equipment, and living spaces. This is shown in the following Heat Transfer Equation:
Q = LHe x m
Where: Q = evaporative heat (heat loss)
LHe = latent heat of vaporization/evaporation of water (Btu/lb.)
m = mass of water
Water is used as the medium in cooling towers. A relatively large quantity of heat is released (1,000 Btu’s) for every pound of water evaporated. When dealing with water, heat transfer is approximately 1,000 times more efficient through evaporation versus sensible heat. The actual value is slightly lower (970.4 Btu’s), but throughout the industry, the 1,000 Btu per pound value is universally accepted.
Cooling towers are designed to create effective heat transfer by connecting air and water in an efficient and expedient manner. Anywhere from 75–95 percent of the heat from the process, equipment, or buildings is removed through evaporation and only 5–25 percent is removed via convection.
The concept of wet bulb and dry bulb temperatures should be considered. Wet bulb temperature is defined as the lowest water temperature at which heat can be removed via evaporation. The lower the wet bulb temperature, the lower the relative humidity, and the more efficient the cooling tower is at heat removal.
There are three different tower designs. The right type is determined based on the size of the system/application, geographical location/climate, water quality, and local utility costs.
When the recirculating water in a cooling tower evaporates, it theoretically exits as a pure vapor. An extremely small percentage of this vapor carries tiny water droplets containing dissolved solids. This is known as cooling tower drift. At this early point in our discussion, cooling tower drift will be considered out of scope.
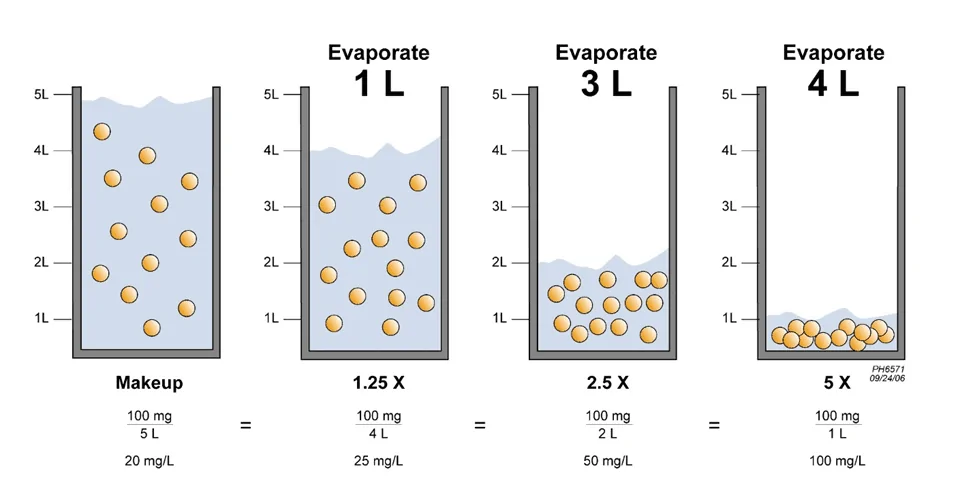
The sequential diagrams in Figure 1 illustrate what we mean by cycles of concentration. As more water is evaporated, the quantity of dissolved solids stays consistent, so their concentration increases.
The equation in Figure 2 helps determine how much water is being evaporated (E) in terms of gallons per minute.
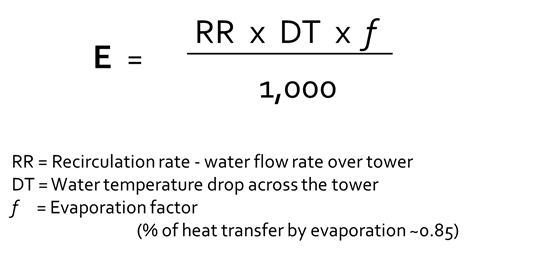
Note: The term ΔT is the actual measured temperature drop across the tower and not the design temperature.
Hot return water and air flow are in direct opposition to each other. Drift eliminators introduce a difficult path for water droplets to navigate. Most water droplets cannot find their way to the atmosphere and fall back into the cooling tower basin, leading to decreased annual water consumption.
Although it is not considered the most efficient, splash-fill is still used in many applications, including poor quality makeup water or towers serving applications with a high potential for cooling water process contamination. Under these conditions, the higher efficiency fill may foul and require frequent chemical cleanings.
Film-fill is most frequently used and widely known. It is the familiar PVC-type construction, with the characteristic waffle pattern that spreads the cooling water over a larger surface area for contact with the circulating air flow. Film-fill can be used in applications where the makeup water quality is good and hot return water temperatures never exceed 140°F.
In addition to the equations below, cycles of concentration (COC) can also be calculated by using the ratio of makeup water to blowdown.
Evaporation (gpm)
Recirculation Rate (gpm) x ΔT x Ef / 1,000
Makeup (gpm)
MU = Evap (gpm) x (C/(C-1))
C = cycles of concentration, commonly determined by either:
cooling tower chloride/makeup water chlorides concentration
or
Cooling tower magnesium/makeup water magnesium concentration
Use magnesium if chlorine, hypochlorite, bromine, or chlorine dioxide is used as an oxidizing biocide.
Cf = Final additive concentration
If possible, adhere to the following recommendations when choosing a cooling tower location:
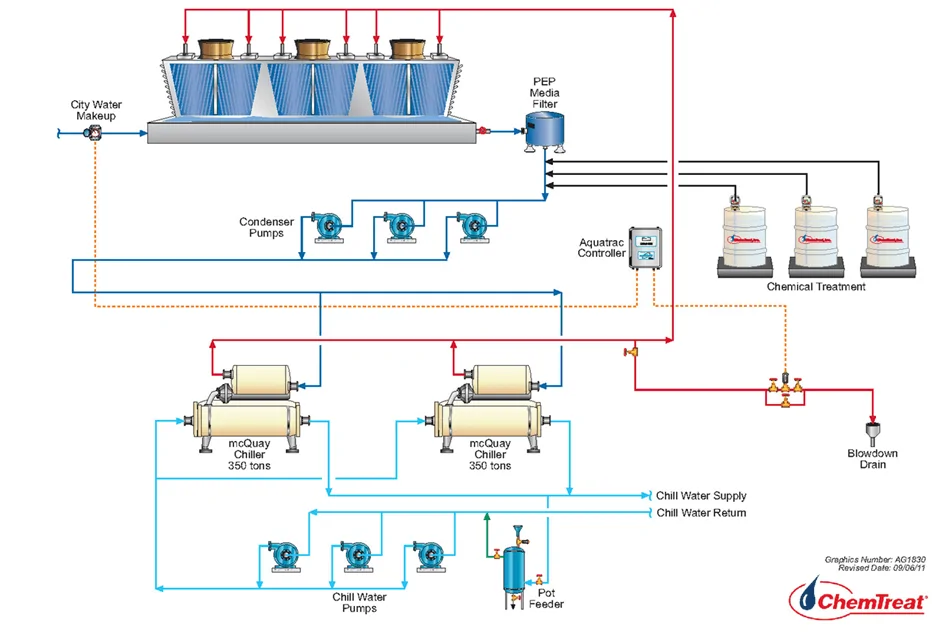
Through manipulation of condenser and chilled water header isolation valves, the system can be set up to have cooling tower water flow bypass the chiller (which is secured/not running) and flow directly through the chilled water piping/coil to the air handler.
The water from the air handler is then returned to the cooling tower to repeat the cycle. Not running the chiller compressor and chilled water loop circulation pumps helps conserve electrical power usage. The required outside ambient air temperature for free cooling is 40–45°F.
It’s important to stay vigilant and keep the following in mind:
Many factors contribute to the efficiency of your cooling system. As with all other technologies, due diligence is necessary when determining the feasibility of utilizing new methods. Always consult your equipment manuals and guides, and don’t forget to contact ChemTreat’s experienced team for assistance!
Senior Technical Support Consultant
Pete Elliott is a trusted technical expert with over 30 years of experience in water treatment solutions for utility and thermal processing systems. In his line of work, Elliott focuses on producing best practices for efficient plant operations, energy and water conservation, and asset protection and preservation. He has published technical papers for the Cooling Technologies Institute (CTI) and the Association of Water Technologies (AWT). Elliott holds a B.S. in Civil Engineering from Villanova University and served as an engineering officer in the United States Navy.
