-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
Film-forming amines (FFAs) have been successfully used in boiler applications to help facilities reduce corrosion, deposition, and iron transport throughout their steam systems.
This innovative technology can be particularly beneficial for refineries, where maintaining utility system conditions is important for production efficiency.
Traditional boiler treatment programs do not generally include a corrosion inhibitor. Pretreatment is used to reduce scale and corrosive ions, and oxygen levels are reduced via mechanical and chemical means. pH is raised to passivate metal surfaces, and temperature is increased to deoxygenate the water through a deaerator, helping build the passive layer.
In complicated systems such as those seen at refineries, these standard processes may not achieve the desired level of corrosion inhibition. Film-forming amines can be a beneficial supplement to the treatment program, as they bind directly to the metal surfaces, where corrosion is typically more difficult to treat.
FFA programs have primarily been used in power industry applications in the past. However, they can offer significant benefits to applications specific to the refining industry.
In combination with an existing corrosion inhibition program, FFA can help:
FFA can help reduce corrosion in the following higher purity systems:
These products also help reduce flow-accelerated corrosion and issues related to layup.
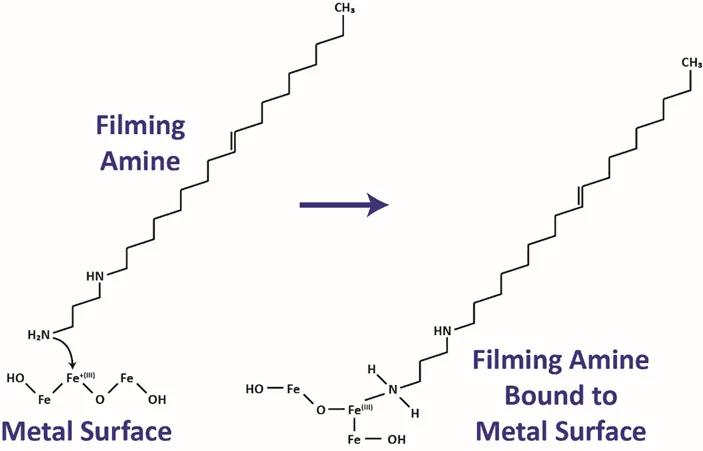
Film-forming amines contain a long chain hydrocarbon as part of the molecule. The amine portion of the molecule allows it to form an attachment to the copper or iron within the oxide layer on the metal surface. The hydrocarbon portion of the molecule repels water, inhibiting water transport to the surface where the film is formed.
In traditional treatment, mild steel develops a passive oxide layer by building layers of magnetite particles that get progressively less organized and irregular in shape as they move away from the surface. The stacking of these particles decreases the corrosion rate so smaller, more organized particles result in decreased layer depth. Particle size is strongly influenced by the solubility and mobility of the magnetite particle as well as the oxidation rate of the corroding ferrous iron from the metal surface.
A low concentration of oxygen increases the oxidation rate of ferrous iron dissolving from the surface, which both increases magnetite formation and allows for the formation of low concentrations of hematite.
Hematite forms an iron oxide polymer that interacts with the magnetite, decreasing its mobility. This reduces the particle size and cements the particle in place, allowing for a more compact, passive, and stable iron oxide layer.
With FFA treatment, the epitaxial layer is depleted, and a film is formed to create a hydrophobic surface. If the FFA residual is decreased below a film maintenance level, the epitaxial layer will regrow to its original depth.
FFA treatment will remove the less organized top portion of the iron oxide layer because it is not bonded with the other particles as effectively. Once the looser iron oxide layer is removed, the film starts to build and increase in density as more treatment is added.
A successfully applied FFA program will create a hydrophobic layer on boiler surfaces.

Image of a steam drum with a hydrophobic layer of FFA. Water droplets stay on the surface of the drum without being absorbed into the surface and causing corrosion.
Prior to feeding FFA treatment in your boiler systems, several factors may need to be considered. These include, but are not limited to:
To monitor the effectiveness of FFA treatment, tracking the following parameters is recommended:
Does ChemTreat feed FFA as a stand-alone program for deposit, scale, and corrosion control?
ChemTreat does not feed FFAs as a standalone program. We feed film-forming amines as a supplement to traditional treatment programs for systems that need an enhanced level of corrosion inhibition.
Does this technology “gunk up” like previous generations utilizing octadecylamine (ODA) chemistry?
ChemTreat’s FFA technology is more volatile than products such as ODA and does not have the same solubility issues. Filming amines can also be tested to verify that product is not being overfed. FFA application has not caused issues such as gunking, probe fouling, strain clogging, etc.
Are filming amines sensitive to pH?
Unlike previous generations of this technology, ChemTreat’s filming amine is not pH-sensitive, so the hydrophobic layer stays intact if pH increases.
Do filming amines bind catalyst sites?
ChemTreat’s FFA technology has been fed in systems where steam directly contacts with catalyst sites, and we have not observed the product binding with catalysts. Catalysts are very active sites that operate at a temperature above the stability of FFAs, so we would not expect filming amines to bind with them.
Is FFA the Right Treatment Solution for my Refinery?
As with all other technologies, due diligence is necessary to determine the feasibility for utilizing the methods discussed in this post.
It is always important to consult your equipment manuals and guides and seek guidance from your local water treatment representative to address your specific needs.
Contact ChemTreat today to see if FFA treatment is right for your facility.
Boiler Product Strategy Consultant
Dale Stuart, Boiler Product Strategy Consultant, joined ChemTreat in 2000. He has an M.S. in Chemistry and two decades of experience in research and development of scale and corrosion control programs for boiler and cooling systems, with a focus on film-forming amines and automation platforms using fluorescence.
Technical Staff Consultant
Kurt Kraetsch is a Technical Staff Consultant with ChemTreat. He has 20 years of experience in the specialty chemical industry, heavily focused on boilers in chemical, refining, and petrochemical facilities. He has an additional 10 years of chemistry and steam plant operational experience serving aboard nuclear submarines in the U.S. Navy. Kraetsch holds a B.S. in Nuclear Engineering Technology from Thomas Edison State University in Trenton, New Jersey.
