-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
By Brad Buecker, ChemTreat, Inc. and Ken Kuruc, Hach
This article was originally published in PPCHEM® Journal; PPCHEM® 2021, 23(4), 152–157; https://journal.ppchem.com/
Industrial facilities such as refineries, petrochemical plants, steel mills, metal finishing facilities, pulp and paper mills, pharmaceutical plants, etc. require substantial wastewater treatment, as some processes at these facilities can release many complex carbon compounds or other toxic constituents, including metals, to waste streams.
While various techniques are available for measuring trace level metals in process water, to date they have been rather unavailable to many industrial locations because of capital cost requirements or the need for specially trained technicians. Two well-known techniques are inductively-coupled plasma and atomic absorption spectroscopy, which need specially trained operators and require complex sample preparation and expensive instrumentation.
This article discusses another existing technology, colorimetry, which has been modified for on-line monitoring. The method is suitable for many facilities and can be operated by a wide range of plant personnel. In many cases, the readings can be enhanced with TOC analyses to provide additional protection for industrial water/steam systems.
When author Brad Buecker began a lengthy career in the coal-fired power industry four decades ago, common discharge regulations for US power plants focused on four impurities or parameters: total suspended solids (TSS), pH, oil and grease (O&G), and residual oxidizing biocide concentration. These days, it is well recognized that many other wastewater impurities can cause problems for power producers. Of course, facilities such as refineries and petrochemical plants require substantial wastewater treatment, as refining and organic chemical synthesis can release many complex carbon compounds and toxic constituents to waste streams. Many other heavy industries such as steel mills, metal finishing facilities, pulp and paper mills, and pharmaceutical plants also deal with challenging wastewater treatment issues.
Along with these applications, discharges from other seemingly benign processes (e.g., cooling tower blowdown and storm water runoff) are facing increased regulation because of their potential negative effects on the natural environment. A well-known example is tighter regulation of phosphorus in cooling tower discharge, which was previously reported in this journal [1]. Ammonia is another impurity of concern; like phosphorus, it is a prime nutrient for the large algae blooms that have plagued many surface water bodies. New technologies that minimize or eliminate inorganic and organic phosphates for cooling tower water treatment have become very popular.
However, concern continues to grow over discharges containing transition and heavy metals, as well as metalloids. These constituents include zinc, copper, chromium, selenium, arsenic, and others. The ability to monitor trace concentrations of metals is important for plant operators and technical staff when evaluating the efficacy of treatment programs and ensuring compliance with discharge guidelines.
While trace concentrations of some heavy metals are important for certain biological functions, others pose a significant danger to the environment and sometimes even to internal plant processes. Antimony, selenium, and arsenic are highly toxic even at very low levels. Chromium, arsenic, cadmium, mercury, and lead have a strong affinity for sulfur, and can bond to enzymes in the human body, inhibiting metabolic reactions. Arsenic, hexavalent chromium, manganese, and cobalt are carcinogens. Cadmium causes a degenerative bone disease. Mercury, lead, and manganese can damage the central nervous system. From an industrial perspective, manganese, aluminum, and iron can cause deposition and corrosion problems in plant process and cooling water systems. This list is not exhaustive, but it offers an indication of how serious the negative impacts of many metals can be [2-4].
Of course, much R&D has gone into treatment technologies for metals removal from process and wastewater streams. We will discuss several techniques before moving to the monitoring portion of this article. Perhaps somewhat surprisingly, many modern treatment methods do not rely on exotic equipment but have resulted from chemistry improvements within traditional processes. Consider the CoMag® ballasted clarification process [5]. Ballasted clarification has become popular, as these units operate with high rise rates, which, in turn, allow for clarifier design with much smaller footprints than conventional equipment. The original material for many ballasted clarifiers was micro-sand, which works well for providing a dense material to capture floc and improve settling. However, this precipitation process utilizes magnetite (Fe3O4) ballast. As with micro-sand, magnetite offers a dense substrate for settling enhancement, but with a significant advantage in that several heavy metals will co-precipitate with the iron and depart the system within the clarifier sludge. These trace metals or metalloids include copper, aluminum, and arsenic.
Another chemistry advancement relies on the aforementioned affinity of sulfur for certain metals. A leading example is mercury, which binds quite strongly with sulfur. Water treatment polymers with active sulfur sites have been developed for blending with other flocculating agents in standard clarifiers. These polymers can be quite effective for mercury removal, but sometimes the reaction is so fast that it generates very fine flocs. Care may be necessary to prevent the fine flocs from escaping with the clarifier overflow.
Another problematic impurity for several industries is selenium. Selenium is a naturally occurring element that, in power plant coal combustion, is typically released in two species, selenite (SeO3) and selenate (SeO4). These oxides are captured in ash sluicing or wet flue gas desulfurization (WFGD) streams. For years, the best available technology (BAT) for selenium removal, as determined by the United States Environmental Protection Agency, has been biological treatment per adsorption of the oxidized selenium compounds on an organic substrate, and subsequent digestion of the selenium oxides by microorganisms that convert the compounds to elemental selenium retained by the microbes. These systems are very large, expensive, and require periodic removal of spent organisms and replenishment of the microbiological substrates.
ChemTreat now offers SeQuester™, which is a physical-chemical alternative to biological selenium removal. The process utilizes co-precipitation chemistry and pH adjustment to capture selenium in conventional wastewater treatment equipment such as clarifiers, holding tanks, and filter presses. The configuration can be designed to treat not only direct discharge from wet scrubbers, but also stored ash pond water, collected landfill leachate, and mine tailing discharges, all at potentially much lower cost than the current BAT methods. Furthermore, pilot test results indicate the technology may lower the concentrations of other trace metals, including arsenic, cadmium, chromium, lead, mercury, and silver [6].
Many coal plants, both operating and shuttered, are also struggling with environmental edicts to close ash ponds. Several well-publicized accidents have released large quantities of ash and water into the environment. Since pond remediation does not allow for the water to be directly drained to some other receiving body of water such as a river or lake, rigorous treatment may be necessary before ponds can be drained and closed.
A critical aspect to successful removal of impurities from wastewater streams is accurate monitoring of the contaminants, which brings us to the second section of this article.
We will now examine an existing and somewhat familiar technique for metals monitoring. The colorimetric method, with an option for digestion to determine both dissolved and total concentrations, has been adapted for on-line trace metal analysis.
Various criteria exist for categorizing metals. Generally, heavy metals are those that have a density greater than 5g ⋅ cm–3. This is the criterion selected for this discussion, even though other metals or metalloids such as aluminum and selenium may be of concern in various processes despite not formally fitting into this category [7]. Often, the transition metals are lumped in with heavier metals when it comes to certain properties.
A variety of technologies are currently available for monitoring trace metal concentrations. However, many methods only offer snapshot analyses of a sample obtained from the process stream of concern. Two well-known techniques are inductively-coupled plasma (ICP) and atomic absorption spectroscopy (AAS), which need specially trained operators and require complex sample preparation and expensive instrumentation.
Such methods may be acceptable when the water sample is not expected to change over short periods of time, e.g., 24 hours. However, in many process streams, upsets or deviations may frequently occur because of changes in some aspect of the overall process. In any of these instances, some type of on-line or continuous monitoring is needed for accurate evaluation of process conditions.
Before moving forward with this discussion, it is important to set forth some qualifications. Colorimetry requires a relatively clean sample, which may preclude the use of this technique in some industrial settings. In general, maximum particle size should be less than 100μm, at a concentration of less than 0.1g ⋅ L–1 and a turbidity (measured as nephelometric turbidity unit, NTU) of less than 50NTU. Total organic carbon (TOC) should be less than 25mg ⋅ L–1. These are meant to be guidelines only and not a guarantee of performance. Sample dilution, either internal to the analyzer or external, may be an option, including when color is present. Filtering is also possible for samples with suspended solids, but consideration should be given to whether the particles being removed contain any of the substances being measured. This being stated, the analytical method will now be described.
Many power plant and industrial chemists have utilized colorimetric techniques for years to track water/steam chemistry. Common parameters include phosphate, silica, and ammonia, with the technology having long ago advanced beyond grab sample measurements to continuous on-line analyses. In fact, applications for continuous phosphate monitoring continue to evolve. One example of this evolution is the increasing selection of reclaimed municipal wastewater as makeup supply for industrial plants. Accurate analyses of inlet phosphate concentrations are important for controlling cooling water treatment programs and other processes.
Grab sample colorimetric analysis for metals has also been a viable method for decades, but adapting colorimetry for on-line monitoring requires additional consideration. This can be accomplished using software-controlled valves that introduce the required reagents at the appropriate time.
The metals that can currently be analyzed with colorimetry are chromium, manganese, iron, nickel, copper, and zinc. While not considered as heavy metals, aluminum and boron, which have been shown to be detrimental to health and the environment at certain levels, can also be measured using this technique.
For those metals that exist as suspended particles, digestion is required to convert them to a dissolved state. Generally, this is accomplished via acid addition and heating to 120°C for a minimum of 10min. This step is performed in a separate digestion vessel per Figure 1. The sample is then cooled, transferred to the analysis vessel, and subjected to an initial absorbance reading taken before reagent addition. The proper analytical wavelength will conform to the specific metal and reagent combination responsible for the color development. The final absorbance is then measured after reaction of the reagent with the metal, with final calculation per Beer's Law.

Figure 1: Digestion unit.
This method is summarized in Figure 2, and the correct chemical reagents vary depending on the metal to be analyzed. For example, adding ethylene-diamine-tetraacetic acid (EDTA) and a reducing reagent minimizes interferences when analyzing zinc or manganese. Some interference may still occur if the sample has large amounts of color, turbidity, and significant concentrations (mg⋅L–1 levels) of certain other metals.
Figure 2: The colorimetric cycle. *If additional buffer is added, ABS1 is only read after addition of this buffer
It should be noted that although colorimetry has evolved into an on-line method, the analyses themselves are batch reactions. Depending on the metal and whether or not a digestion is required, analysis times can range from 10 to 30 minutes. While the use of an integrated sequencer can permit the analysis of up to 8 individual streams, the number of discreet readings per stream is reduced accordingly (e.g. with a 10-minute cycle time and 6 streams, only 1 reading per stream is reported per hour). A typical instrument with software-controlled valves, digestion vessel and colorimeter is illustrated in Figure 3.

Figure 3: Hach EZ Process Analyzer.
Another on-line monitoring parameter receiving increased and deserving attention is total organic carbon. As Figure 4 suggests, TOC data from numerous plant processes can be valuable.
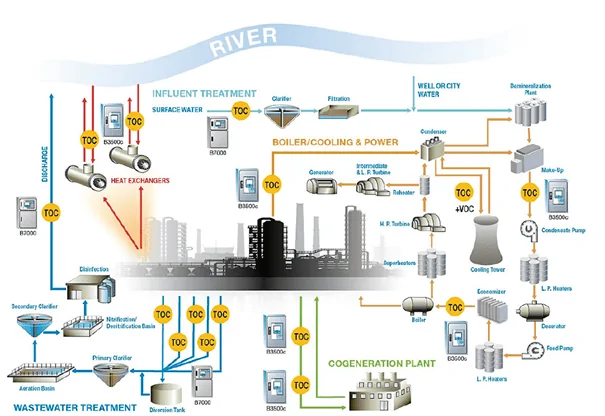
Figure 4: Potential TOC sampling sites at an industrial facility (illustration courtesy of Hach).
Naturally, at plants such as refineries and petrochemical facilities, continuous TOC monitoring of condensate return and other process water streams can alert personnel to leaking heat exchangers and other equipment. For example, one of the authors relatively recently visited a liquefied natural gas (LNG) import facility that was being converted into an export facility. A major part of the process is removing hydrocarbons larger than methane from the incoming natural gas before liquefaction. The condensate return lines to the co-generation plant have TOC analyzers to detect organic contamination that might enter from this and other processes.
Consider some of the other sample points in this diagram. TOC could be a front-line indicator of potentially harmful organic compounds leaving the plant in a wastewater stream. If the plant has a non-fresh water source for plant makeup, such as reclaimed municipal wastewater effluent, TOC could again be a front-line indicator of upset conditions at the municipal wastewater plant. It is not unknown for plants that handle combined wastewater and storm runoff to be overwhelmed during heavy rains and need to bypass some wastewater that has received only primary treatment. Organic carbon levels can increase dramatically in these conditions. An increase in TOC to cooling tower makeup can upset cooling water chemistry, cause difficulties in high-purity makeup treatment systems, and, most importantly, influence microbial growth in heat exchangers, cooling tower fill, and other locations.
While various techniques are available for measuring trace level metals in process water, to date they have been rather unavailable to many industrial locations because of capital cost requirements or the need for specially trained technicians. This discussion outlined an existing technology that has been modified for on-line monitoring. This method is suitable for many facilities and can be operated by a wide range of plant personnel. Additional R&D continues exploring instrumentation for analyzing other metals of concern in wastewater processes. In many cases, these readings can be enhanced with TOC analyses to provide additional protection for industrial water/steam systems.
Of course, all systems are different, and, as with all other technologies, due diligence is necessary to determine the feasibility of utilizing such methods. Always consult your equipment manuals and guides.
Contact us to learn more and request a consultation.


