-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
While pretreatment plays a vital role in ensuring reverse osmosis (RO) system efficiency, the chemistry and constituents found in the makeup water are just as important to the system’s overall health.
This article discusses the characteristics of various makeup sources and outlines processes for analyzing RO makeup water.
Read on to:
To understand the potential vulnerabilities of an RO system, it is important to know its makeup water source.
RO manufacturers typically suggest cleaning systems every 3–4 months; yet, in many cases, RO systems require more frequent cleanings. This is, in part, because of the differences in water coming from varying makeup sources, and more importantly, the performance of the upstream filtration equipment. Pretreatment is a key component of reliable and efficient RO.
Makeup water quality is very important. Any imperfections in the water can lead to fouling, scaling, and corrosion if not treated properly.
Surface water is drawn from sources such as:
Typically, all surface waters contain fluctuating levels of suspended and colloidal particles, organics, microbiological activity, and turbidity. If the makeup source is a river, for example, the clarity or turbidity will change based on rainfall and land runoff.
Surface water contains suspended and colloidal particles, which will pass through a 5-micron cartridge filter in the RO, increasing pressure changes (ΔP). This can result in RO fouling during the first stage. Conducting a Particle Size Analysis of the makeup water and the outlet of the cartridge filter housing is recommended at this stage.
Another source for makeup is well water.
Generally, the deeper the well, the cleaner the water. Deep wells have more consistent temperatures, and water pulled from these wells often contains lower levels of bacteria, colloidals, organics, and suspended particles.
Gray water typically refers to tertiary wastewater intended for municipal and industrial reuse.
In recent years, water scarcity has made gray water a more popular source for makeup. Instead of using surface water, many municipalities now use gray water from power plants, chemical plants, and refineries for their processes.
Although gray water use has gained in popularity, it comes with unique treatment challenges. Gray water typically has a large amount of organic loading, ammonia, and phosphates, while its low chlorine residual is rarely sufficient for removing microbio.
Silt density index (SDI) testing is a best practice for analyzing RO makeup, as it measures the fouling capacity of the water used in the RO.
SDI testing is performed on site with an SDI kit. The test requires a constant makeup stream at 30 psi. This stream is passed through a 0.45-micron filter pad at a known volume for 15 minutes to calculate SDI.
Typically, RO manufacturers recommend an SDI of 5 or below, but many are now indicating that 3 or less is needed for good RO performance.
In most cases, the lower the SDI, the better the system will run. It is a good idea to save the SDI pads for historical reference.
Utilizing surface water as a makeup source requires facilities to implement either a clarification process to remove turbidity or a cold lime softening process to reduce hardness, alkalinity, and turbidity.
Regardless of which process is implemented, treatment will include coagulant and/or flocculant chemicals.
Coagulants have one of three bases:
The amount of coagulant fed to the system greatly varies based on the surface water source.
A good example of this is the Mississippi River, which flows north to south. At the top of the river in Minnesota, the turbidity level of the water is low, so coagulant feed will be in a low parts per million (ppm) range.
As it moves south, the Mississippi meets the Missouri, Illinois, and Ohio rivers. These confluences lead to turbidity formation, so water treatment plants along the river feed higher amounts of coagulant into their system to adjust for higher turbidity.
Flocculants also play an important role in turbidity reduction. They can be cationic, anionic, or non-ionically charged. Flocculants are typically dosed at less than 2 ppm, so it is important to monitor how flocculants are being added to your system.
Though coagulants and flocculants are beneficial for producing the desired finished water quality, they can still be detrimental to overall RO membrane health.
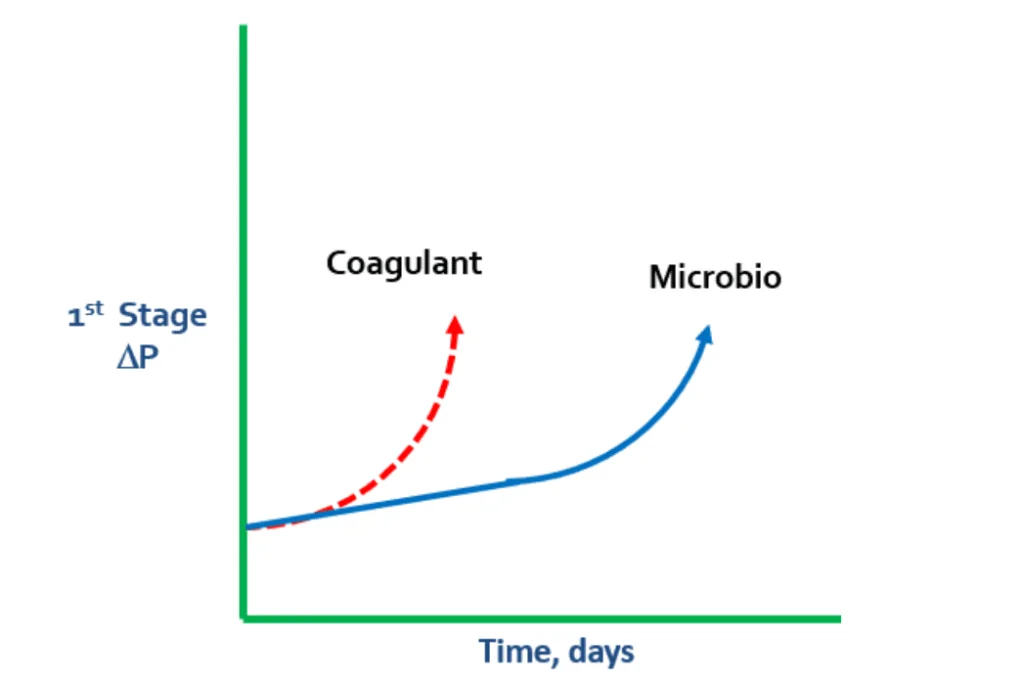
Unlike the slightly negatively charged RO membranes, coagulants are cationically charged, which can cause pressure changes. If there is a rapid spike in ΔP during the first stage, it is prudent to look for a coagulant problem. Coagulant levels may not have been adjusted to match changes in turbidity, which can result in coagulant overfeed.
In these cases, coagulant begins to lay down on the membrane and grab microbio, particles, and colloidals. When the negatively charged bacteria meets the positively charged coagulant, the coagulant continues to grab more and more microbio.
In some cases, extreme levels of polysaccharides in microbio can cause a “flypaper” effect, which leads to microbiological matter grabbing suspended solids and colloidals from the water. This can result in membrane fouling.
If the RO membrane is not cleaned properly, coagulant issues can lead to gap formation.
Once gaps form inside an RO membrane, they are very difficult to remove. Therefore, it is important to monitor microbio levels within the makeup water as it enters the system.
Additionally, if a plant is using city water for makeup, it is recommended that facility personnel contact the municipality and ask what type of coagulant has been used upstream.
It is also important to be aware of the mineral contents of makeup water.
Minerals can be broken down into two separate subgroups: cations and anions.
Examples of each are included below:
| Cations | Anions |
|---|---|
| Calcium | Total Alkalinity |
| Magnesium | Chlorides |
| Sodium | Sulfates |
| Potassium | Nitrates |
| Barium | Fluoride |
| Strontium | Orthophosphate |
| Aluminum | Silica |
| Iron | |
| Manganese |
Testing makeup water for mineral content is an essential step in determining the type of RO antiscalant to use and the percent RO recovery rate.
Of the cations, barium, aluminum, iron, and manganese are particularly important to monitor. Levels of each should typically not exceed 0.05 ppm (50 ppb).
Aluminum can be especially problematic. It is typically fed as a coagulant to reduce turbidity, but excess aluminum can lead to RO fouling, which can be difficult to treat with an antiscalant.
The following anions: total alkalinity, orthophosphate, fluoride, and silica, may also need to be monitored closely. Gray water is particularly prone to supplying excess phosphates.
The recommended testing frequency for minerals in makeup is:
In general, calcium phosphate, calcium sulfate, and barium sulfate can all be treated with an antiscalant, while aluminum, iron, and manganese may be a bit more difficult treat.
Once the pH and total alkalinity are known, the amount of free carbon dioxide (CO2) can be calculated.
Because it is a gas, CO2 passes through the RO membrane, which can lead to pH reduction in the permeate.
Since CO2 is not detected by a conductivity meter, it can flow downstream to the mixed bed and result in a large amount of hidden loading. This could require more frequent replacement of the mixed bed.
Calculating conductivity provides an idea of the number of ions present in the water. For example, high conductivity (300–500 µS) may indicate that hardness and alkalinity levels are within range, but there may still be a high number of chlorides or sulfates in the water. High conductivity may also suggest that more permeate is traveling downstream to the softener or other ion exchange unit.
Surface waters are notorious for having high levels of organic matter, which is measured as total organic carbon (TOC). For RO feedwater, the TOC limit is 3 ppm.
Organics with a molecular weight of 150–200 ppm and higher are rejected by the RO; however, a certain percentage of organics below that molecular weight will pass through the RO into the permeate.
Temperature affects the flux rate for permeation. Cooler temperatures cause membrane pores to tighten, decreasing the flux rate. Warmer water loosens up the pores, allowing more total dissolved solids (TDS) or ions to pass through.
Orthophosphates in gray water or calcium in industrial reuse makeup streams can form calcium phosphate (a sludge-like deposit) in the last stage of the RO as the water gets warmer.
The solubility of silica and the flux, on the other hand, decrease as water temperature goes down, and vice versa.
Chlorine degrades RO membranes and needs to be removed either with uncatalyzed sodium bisulfite or an active carbon filtration system. If a transitional metal, such as iron, manganese, or cobalt, is present in a system containing chlorine, the rate of membrane degradation is accelerated.
Often, active carbon beds are paired with a biocide program. The two most commonly used in the industry are DBNPA and isothiazoline. These biocides help alleviate the microbiological activity on the membrane surfaces and the feedwater spacer, where bacteria are easily attached, thus reducing the potential of bacteria entering the system and feeding on the organics.
Chlorine is typically measured with an ORP in-line meter or a chlorine analyzer prior to entering the RO. It is a good practice to wet test for chlorine in addition to using on-line instrumentation.
Turbidity below 0.5 Ntu is a good target for makeup water, but low turbidity does not necessarily indicate the reduction of fouling potential; SDI testing provides more reliable insight.
If it is suspected that colloidal particles are entering the RO, run an SDI pad until it is plugged up and send the pad to the laboratory for a scanning electron microscope analysis. This test will indicate the characteristics of the atomic elements on the SDI pad, providing insight into what is fouling the RO.
COD is found in gray water or industrial reuse and can be a major nutrient source for microbio.
A COD range of 8–10 mg/L is a good indication that the makeup source will have low fouling potential from COD.
Two types of bacteria can be present in ROs: aerobic and anaerobic.
Classified by two types: planktonic and sessile
Sessile bacteria are developed when aerobic bacteria are allowed to multiply and begin adhering to piping and membrane surfaces. This results in the aforementioned “flypaper” effect.
Best practice is to maintain microbio at 100 Cfu/mL in the feedwater and 1,000 Cfu/mL in the RO reject.
Normally, H2S is found in wells and is notable for its strong rotten egg odor. If oxidized or exposed to air, H2S can form elemental sulfur in the lead membrane, leading to potential blockage. To maintain system efficiency, the maximum level of H2S in RO feedwater is <0.1 ppm.
Hydrogen sulfide must be tested for on-site.
To test for H2S, fill up a water bottle halfway with makeup water, add a couple drops of hydrochloric acid into the bottle, shake, take the cap off, and smell. If hydrogen sulfide is present in the water, the odor will be apparent.
As previously mentioned, the lower the alkalinity, the higher the CO2 content. Fortunately, CO2 does not play an active role in RO scaling or fouling. It passes directly through into the permeate.
It is important to monitor pH and alkalinity because they provide an indication of how much CO2 is flowing through the RO membrane. High amounts of CO2 can place a large load on the strong base anion and mixed bed.
Ammonia is typically found in gray or industrial reuse makeup streams.
At a pH below 8.5, 90% or more of the ammonia is in its ionic form as NH4- and will be rejected in the RO like a monovalent ion. Above pH 9.5, ammonia becomes gaseous.
When mixed with TOC or phosphates, ammonia becomes suitable food for bacteria.
To test for ammonia, grab a water sample, add a few drops of 50% caustic, and shake the sample. If ammonia is present in the water, the strong smell will be apparent.
When using surface water for makeup, testing for microbio, organics, suspended solids, and colloidals is recommended. Season changes and shifts in weather conditions can cause rapid fluctuations in the levels of these components, so it is important to test frequently.
Though well water quality is generally considered consistent, semi-annual testing is recommended, as it can have high mineral and hydrogen sulfide levels as well as some bacteria.
Various foulants in gray water may cause issues in RO systems, so frequent testing is recommended. It is important to test for bacteria and wash the cartridge filter housing regularly.
Regardless of the makeup water source, we recommend trending water chemistry and other constituents and keeping trends on file. This data can assist in troubleshooting if RO problems arise.
As with all technology, due diligence is important to determine the feasibility of utilizing the methods outlined here. Always consult your equipment manuals and seek guidance from your water treatment representative to address plant-specific needs.
 Meet the Expert:
Meet the Expert:Director; Filtration, Ion Exchange & Membrane Technologies
Ed Sylvester started working in water treatment in 1976 while serving in the U.S. Navy. As Director of Ion Exchange and Membrane Technologies at ChemTreat, Sylvester has received company recognition for his involvement in energy savings projects and his support of customers in the ethanol, hydrocarbon, chemical, and middle market industries. His areas of expertise include pretreatment (membrane filtration, ion exchange, and clarification) and high-pressure boiler treatment.
