-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
By Brad Buecker, Senior Technical Publicist
High-purity makeup water production and precise boiler water/steam chemistry control are critical for high-pressure steam generators in the power industry. However, many industrial plants utilize low-pressure boilers to produce process steam for various applications, including heat for chemical reactors, evaporators, building spaces, and so forth. Even though treatment programs for these units are often considered to be less rigorous than for high-pressure boilers, plant personnel frequently underestimate the corrosion and scale-forming difficulties that result without proper selection and operation of makeup water and condensate return treatment systems.
For lower-pressure steam boilers (units up to approximately 600 psi that do not drive turbines), makeup water treatment is often not extraordinarily rigorous. Usually, the leading concern is the potential for calcium carbonate (CaCO3) scaling, as illustrated by the following reaction of calcium ions (Ca2+) and bicarbonate alkalinity (HCO3- that can occur in hot water systems and boilers.
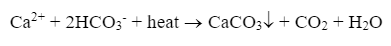
CaCO3 is the whitish-tan material that appears in home hot water piping and shower heads and is often incorrectly referred to as "lime scale."

For decades, a typical primary treatment method for industrial boiler makeup was sodium zeolite softening. In this process, the water passes through beds of ion exchange resin, where the hardness ions calcium and magnesium are swapped for sodium. The softened stream, with the remaining impurities including alkalinity, chloride ions (Cl-), sulfate ions (SO42-), silica (SiO2), etc., then feeds the boiler.
This straightforward method offers both advantages and drawbacks. For example, the single process of sodium softening, as compared to those makeup techniques needed for high-pressure steam generators (for example: ultrafiltration, reverse osmosis, and RO effluent polishing) save the plant money in equipment and operating costs. But sodium softening does not touch the other ions, many of which can be problematic. Alkalinity may convert to carbon dioxide (CO2) in the boiler, which then carries over with steam. The CO2 can lower the pH in condensate return, possibly inducing corrosion issues in these systems. Chloride, and to a lesser extent sulfate, can be nasty impurities, especially in combination with oxygen in the boiler. The compounds may also concentrate under porous boiler tube deposits, usually iron oxide corrosion products transported from elsewhere, e.g., condensate return systems, to induce under-deposit corrosion (UDC).
One final point to consider: over the years, ChemTreat personnel have seen many instances of scale formation and corrosion in steam-generating systems, where the primary problem was traced back to upsets or poor maintenance of sodium softeners and other makeup treatment equipment, which allowed excessive impurities to enter the system. The water/steam plant seems to be neglected until problems arise, and the same is often true with regard to controlling condensate return chemistry.
As noted previously, sodium softening has served as the core of many makeup water treatment systems. However, the technology does not remove other ions from the water, including alkalinity, chlorides, sulfates, and silica, to name the most prominent. Several methods are available to improve makeup water purity beyond basic sodium softening. Some of the older, established technologies are:
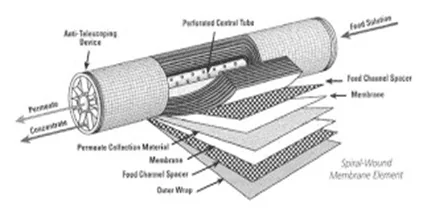
In large measure, these methods are from a bygone era, particularly the first two. The development and maturation of membrane technologies, and particularly reverse osmosis (RO) have altered the landscape. Single-pass, and especially two-pass RO, can produce makeup water with very low dissolved solids concentrations.
Figure 1. Cutaway view of an RO membrane
This Photo by Unknown Author is licensed under CC BY-NC
When pressure is applied to the makeup water feed to RO membranes (usually several series of membranes encased in individual pressure vessels), the membranes reject most of the dissolved solids (up to 99% or greater for modern membranes), to produce a significantly purified effluent (permeate) as makeup for the steam generator.
Keys to successful operation of RO units are pretreatment to remove suspended solids ahead of the RO membranes, and optimized chemical treatment to minimize scale formation on the membranes. Careful analysis of RO feedwater is critical for proper pretreatment equipment and chemical selection. Consultation with reputable RO suppliers and consultants is also necessary to select the proper design.
Use of higher purity makeup water as boiler feed can often reduce the complexity of boiler water treatment.
One major concern with systems whose makeup is treated primarily by sodium softeners alone is the accumulation of silica in the boiler. Silica chemistry, and its reactions with other elements, most notably magnesium and calcium, is rather complex. If insufficient hydroxide alkalinity is present in sodium-softened boiler water, and hardness breaks through from the softener, tenacious silicate scales may form in the steam generator. These insulating deposits reduce heat transfer and boiler efficiency, and may also lead to tube failures because of overheating. Protection from such scale formation is offered by high pH from hydroxide alkalinity, but this increases the potential for under-deposit caustic corrosion and potential tube failures from that mechanism. Minimization of silica transport to the boiler allows moderation regarding hydroxide alkalinity requirements.
Another concern, as has been hinted at before, is the presence of bicarbonate alkalinity (HCO3-) in boiler water from makeup systems with just sodium softeners. Some of this alkalinity reverts to carbon dioxide (CO2) at boiler temperatures, where the CO2 then flashes off with steam. The carbon dioxide can then re-dissolve in condensate return systems.
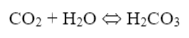
The resultant carbonic acid (H2CO3) lowers the pH of the condensate, which in turn can initiate general corrosion of carbon steel.
The upshot of this discussion is that more modern techniques are possible for makeup water preparation for low-pressure boilers. Like all other technologies, due diligence is necessary to determine the feasibility for utilizing these methods.
Many heavy industries, including refineries, petrochemical plants, pharmaceutical facilities, and steel mills, require steam at numerous locations and have extensive steam feed and condensate return systems with many steam traps, condensate receiving tanks, and miles of piping. Makeup water treatment to the steam generators is often limited to sodium softening, which allows all other impurities in the raw makeup to enter the boilers.
Typically included in these impurities is bicarbonate alkalinity (HCO3-), which, when subjected to boiler heat, decomposes to carbon dioxide (CO2) that exits the boilers with steam. As the steam condenses in various process heat exchangers and other locations, the CO2 re-dissolves and lowers the condensate pH, subjecting condensate return piping and other equipment to acidic conditions that can be quite corrosive.
In the power industry, the common feedwater pH-conditioning chemical is ammonia, which raises the pH via the following reaction:
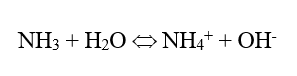
This is an equilibrium reaction, so the alkalinity increase is limited, usually minimizing excessive steel corrosion in the event of a chemical feed upset. (Copper alloy corrosion is a completely different story.) But ammonia is volatile, and the compound significantly partitions with steam in low-pressure boilers.
For industrial units, neutralizing amines are a common alternative for feedwater pH conditioning. These are small-chain organic molecules with an ammonia group attached to or embedded within the compound.
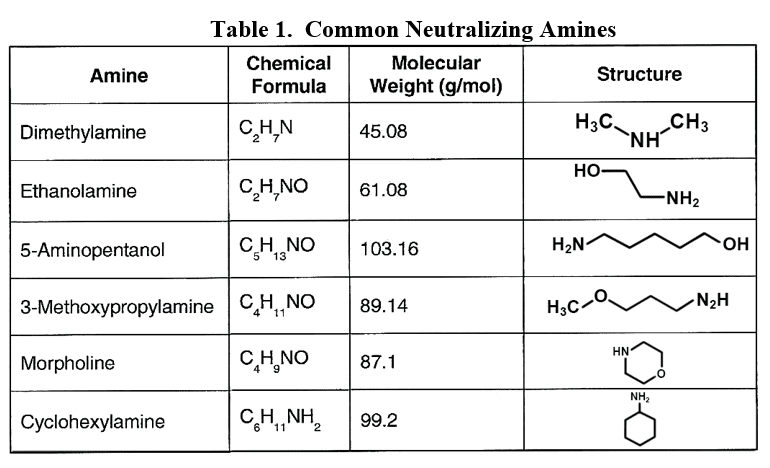
Developing the best program has sometimes been difficult with these compounds, as each has a different basicity and distribution ratio (the tendency for the product to depart with steam or remain dissolved in the boiler water). Many industrial steam generation/condensate return systems are quite complex. It is desirable to have proper pH control throughout the network, but a single compound is often insufficient to achieve these results.
With that very important concept in mind, ChemTreat researchers have developed blended amine products that can provide wide-ranging system coverage from the high-temperature boilers to process heat exchangers to the condensate return piping. ChemTreat representatives can perform plant audits, and, in conjunction with plant personnel, develop a program that fits the needs of the facility. Such programs can be coupled with advanced automated feed and monitoring systems to relieve burdens on plant operators and technical staff.
Condensate return at industrial facilities may contain numerous other impurities, which are in large part dependent on the process chemistry in the heat exchangers to which steam is applied. Even minor tube, plate, or jacket leakage may cause serious condensate contamination. Some form of condensate polishing may be necessary to clean this water before return to the steam generators.
In the early 20th century, as steam-based power generating and industrial units increased in number and size, tri-sodium phosphate (Na3PO4, aka TSP) became a popular boiler water conditioning chemical for drum units. In the utility industry, phosphate treatment programs have undergone much evolution, with TSP used only in low dosages for modern units. For industrial boilers, phosphate treatment methods, with supplemental chemistry, remain a strong choice.
A primary function of phosphate is to generate moderately alkaline conditions in the boiler to minimize general corrosion of carbon steel boiler tubes, drums, and headers.

Although TSP is the only recommended phosphate species for utility boilers (to minimize the potential for acid phosphate corrosion), in industrial units TSP may at times be blended with lesser amounts of di-sodium phosphate (Na2HPO4) and perhaps, although not often, even a bit of mono-sodium phosphate (NaH2PO4) to control excess formation of sodium hydroxide (NaOH), aka caustic. Caustic can concentrate underneath porous boiler tube deposits and induce direct corrosion of the boiler metal.
A second function of phosphate; particularly important for units in which hardness ions may periodically ingress, is to control scale formation. Phosphate and the alkalinity produced by its reaction with water can react with hardness ions and silicates to form soft sludges that can be removed by boiler blowdown.
This is where things get interesting for industrial boilers. While utility units need high-purity makeup, the makeup and condensate return to industrial boilers may have significant impurity concentrations. Thus, in addition to phosphate treatment, sludge conditioners are usually recommended. Sludge conditioners consist of water-soluble polymers that help keep solids in suspension by a combination of crystal modification and sequestration. These polymers can sometimes serve as a stand-alone treatment, particularly if hardness ingress is not an issue.
Another technique that has at times been successfully employed in industrial drum units is chelant chemistry, in which the chemicals directly bind with metals to keep them suspended. Ethylenediaminetetraacetic acid (EDTA) is the most widely known chelant and has been used for many applications, but improper chelant use can cause localized corrosion of boiler components.
The upshot is that several possibilities: phosphate/polymers, polymers alone, and chelating agents, exist for boiler water treatment. The proper choice of treatment depends on a variety of factors that include boiler design and pressure, makeup water treatment sophistication and reliability, and the potential for impurity ingress from condensate return. In some applications, and most notably for the food and beverage industries, FDA regulations limit or restrict the use of some boiler water and feedwater treatment chemicals. This can make the choice of a program more complex.
Also emerging are treatment methods based on film-forming products (FFPs), which provide a protective layer on steam generator metal surfaces. This chemistry could potentially be a game-changer in some applications.
Several choices are possible for the internal treatment of industrial boilers, and these choices are influenced by a number of factors.
Please contact ChemTreat for assistance in designing a treatment program customized for your application. Like all other technologies, due diligence is necessary to determine the feasibility for utilizing methods. Always consult your equipment manuals and guides.
