-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
Steam generation and use for power production and process heating remains a vital unit operation at many power plants and thousands upon thousands of industrial plants around the globe. Chemistry upsets can cause significant damage to equipment in steam generation systems, sometimes in a very short period of time.
Continuous on-line water/steam chemistry monitoring at critical locations in the steam generating network is typically recommended for detecting chemistry upsets and ensuring chemical treatment programs are controlled within proper ranges. This series of posts will examine the primary sample points and their relationship to water/steam chemistry. This post examines the high-pressure units for power generation, and we will discuss lower-pressure industrial steam generators in the next portion of the series.
With the decline of coal-fired power generation and the upswing in renewable energy, a large bridge between the two has been and continues to be simple- and especially combined-cycle power generation with natural gas as the primary fuel. The next several posts will examine primary monitoring parameters for the most common type of heat recovery steam generator (HRSG) from the combined cycle industry: the triple-pressure HRSG, including both the feed-forward low-pressure (FFLP) type and the stand-alone low-pressure (SALP) type.
Note: Low-pressure refers to just the first circuit in these multi-pressure units. Steam for turbine powering comes from the intermediate- and especially high-pressure circuits, commonly termed evaporators, and thus makeup water and feedwater purity must be designed for high pressures and temperatures.
The samples of primary importance throughout either of these designs are:
The discussions will include the normal upper limit, or range, for most of the monitoring parameters. This data and many other details may be found in published documents by the Electric Power Research Institute (EPRI), though they are available only to EPRI members. The International Association for the Properties of Water and Steam (IAPWS) offers technical documents with similar, albeit more condensed, information, which are freely downloadable from their website: www.iapws.org.
Even in the tightest steam generators, a small amount of process water/steam escapes continually. These losses must be made up with high-purity water. The most common core makeup system process is reverse osmosis (RO), followed by either mixed-bed ion exchange (MBIX) or electrodeionization (EDI) to polish the RO effluent. RO units typically include a number of instruments to monitor system performance, including pressure, temperature, flow, pH, and specific conductivity, which are a subject for a separate discussion. The list below outlines the recommended upper limit for the three recommended sampling parameters of the makeup system effluent.

An on-line sodium analyzer. Photo courtesy of Hach.
These measurements ensure high-purity water is continuously being distributed to the steam generators. A rise in any of the values indicates that either the MBIX resin has reached exhaustion or a problem has occurred in the EDI unit. Prompt corrective action is necessary.
Look for subsequent posts as we proceed through the steam-generating network and evaluate critical sampling points and parameters.
Part 2 continues the series on recommended water/steam chemistry monitoring guidelines for high-pressure steam generators. The focus here is on the condensate system. Feedwater monitoring is closely related and will be examined in the next article of this series.
In steam-generating power units, the primary location for potential contaminant ingress is the condenser, particularly water-cooled condensers where a tube leak(s) allows cooling water to infiltrate the high-purity condensate. Cooling water in-leakage will introduce many impurities to the steam generator, which, when subjected to the harsh environment in the boilers (the common term for HRSGs is evaporators) can cause serious problems.
Recommended continuous condensate pump discharge (CPD) analyses are:
Sodium monitoring is very effective for detecting condenser tube leaks. With a tight condenser, sodium levels in the condensate are normally very low (<2 ppb), in many cases, less than 1 ppb. A rise in sodium provides the earliest indication of a condenser tube leak.
Cation conductivity has been re-designated by some research organizations as conductivity after cation exchange (CACE) to illustrate the fact that the sample is routed through a cation exchange column to replace the cations, e.g., ammonium, sodium, calcium, etc. with hydrogen ions. This creates a very dilute acid solution of primarily trace amounts of chloride and sulfate ions, whose conductivity is then measured. As with sodium monitoring, a rise in CACE indicates impurity in-leakage. CACE can be influenced by carbon dioxide ingression, usually from air in-leakage at the condenser. Becoming increasingly popular is degasified CACE, which utilizes either a re-boiler or nitrogen sparging compartment to remove up to 90% of the carbon dioxide.
Dissolved oxygen (DO) analyses are important for monitoring condenser air in-leakage. A sudden increase in dissolved oxygen may indicate a mechanical failure at or near the condenser, which allows excess air to enter the system. Given that modern condensate/feedwater chemistry programs require tight control of DO concentration, this monitoring parameter is very important.

A packaged dissolved oxygen analyzer. Photo courtesy of Hach.
Regarding the relationship between specific conductivity and pH, ammonia (or sometimes an amine or ammonia/amine blend) is the normal pH-conditioning agent for condensate/feedwater. However, direct pH measurement of high-purity water can be tricky, and algorithms have been developed to calculate pH based on SC and CACE measurements to provide more accurate results. SC in high-purity water is directly correlated to the ammonia concentration, so SC measurements offer better control of ammonia feed than pH.
A parameter not typically monitored continuously, but which can be of some importance is total organic carbon (TOC). For utility steam generators, the recommended TOC limit in the CPD is 100 ppb.
Finally, an increasing number of new plants are being equipped with an air-cooled condenser (ACC) as a water conservation measure, reducing issues with cooling water ingress to the condensate. However, because air is so much less dense than water, an ACC should correspondingly be that much larger, which literally requires many thousands of feet of carbon steel piping. The principal condensate contaminant in units so equipped is iron oxide particulate. Some type of particulate filter is recommended to prevent iron oxides from reaching the steam generator. Look for a separate post discussing this issue.
ChemTreat has experience with steam generator water chemistry monitoring and treatment. Contact us for assistance in designing a treatment program customized for your application.
Like all other technologies, due diligence is necessary to determine the feasibility for utilizing methods. Always consult your equipment manuals and guides.
This blog series focuses on different aspects of steam generator chemistry monitoring. Part 1 discussed high-pressure system monitoring recommendations, and Part 2 examined the condensate system.
This discussion on feedwater sampling in steam generator water and steam chemistry monitoring is closely related to the previous installment, especially regarding the recommended instrumentation. The circuit in high-pressure steam generators, and particularly power producing units, is nearly closed (or should be), with only moderate makeup addition. If the condensate chemistry outlined in Part 2 is in order, the feedwater chemistry should also be satisfactory. However, the feedwater sample (ideally at the economizer inlet [EI] or even better, the economizer outlet) is critical, as it typically represents the primary location for evaluating flow-accelerated corrosion (FAC) in the feedwater system. Furthermore, condensate/feedwater treatment chemicals are added after the condensate pump discharge (CPD), so the feedwater sample is the prime location for monitoring chemical dosages and their effects.
For standard systems, recommended continuous feedwater analyses are:
The sodium and CACE samples often serve as excellent backups for the same instrumentation at the CPD. Detection and response to contaminant ingress is typically the highest priority in high-pressure units, and redundant readings from the feedwater instruments can be quite beneficial in confirming whether an upset is real or the result of a faulty analyzer. Although rare, at times impurities have been known to enter the steam generator through contaminated feedwater treatment chemicals, which these instruments would typically detect. Finally, in most high-pressure units, a small slipstream of feedwater is utilized for steam attemperation. This arrangement can introduce impurities directly to the superheater/reheater and turbine. Sodium hydroxide, chloride, and sulfate in particular can be very damaging to these components.
As mentioned in Part 2, ammonia (or sometimes an amine or ammonia/amine blend) is the normal pH-conditioning agent for condensate/feedwater. However, direct pH measurement of high-purity water can be tricky, and algorithms have been developed to calculate pH based on SC and CACE measurements to provide more accurate results. SC in high-purity water is directly correlated to the ammonia concentration, so SC measurements offer better control of ammonia/amine feed than pH.
Virtually no modern steam generators, especially HRSGs, have copper alloys in the feedwater system. Thus, oxygen scavengers/reducing agents are not recommended for these units, but a small amount of DO (5–10 ppb) is necessary to establish the most protective iron oxide layer on carbon steel piping. To do so may require closing deaerator vents (for those systems so equipped), but not without proper upfront evaluation.
In recent years, iron monitoring has become a very important function in many systems. It provides direct indication of FAC and how effective the chemistry program is in mitigating this corrosion mechanism. Most iron in condensate and feedwater exists in particulate form, so analytical methods for only dissolved iron grossly underestimate the total concentration. Corrosion product sampling is a method that filters a sample over time, after which the concentration is determined by digestion of the filter, with subsequent dissolved iron analysis. Hach offers an inexpensive grab sample method, which includes a digestion step, for iron monitoring. Analyses can be performed daily, weekly, or at any frequency desired by plant personnel, and on any of several important streams including condensate, feedwater, and boiler water.
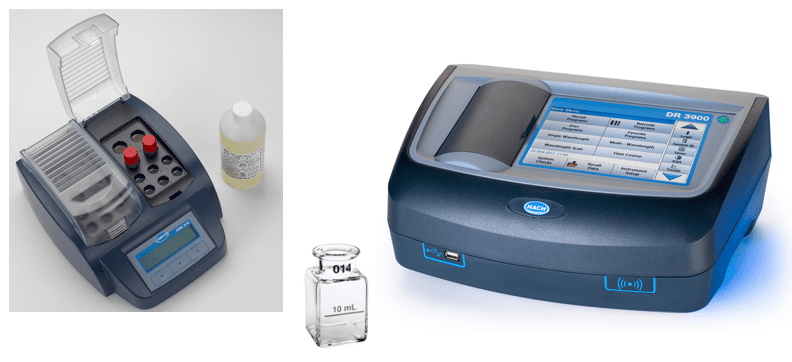
Figure 1. Combination reagent, digestion vials, and heater block (left); 1-inch sample cell (center), and spectrophotometer (right). Photos courtesy of Hach.
ChemTreat can help you address challenges around steam generator chemistry monitoring. Contact us for assistance in designing a monitoring program customized for your application.
Parts 1, 2 and 3 of this series focused on makeup water system and condensate/feedwater monitoring for high-pressure steam generators, and primarily those for the power industry. However, many thousands of lower-pressure boilers exist at industrial plants around the globe, and these also require accurate monitoring and chemistry control. In this post, we will focus on monitoring water and steam chemistry in lower-pressure boilers, specifically issues with condensate return.
A complicating factor at many industrial facilities is the often large and complex steam feed/condensate return network. Depending on the products manufactured at a facility, any number of impurities can potentially enter condensate return and be transported to the boilers.
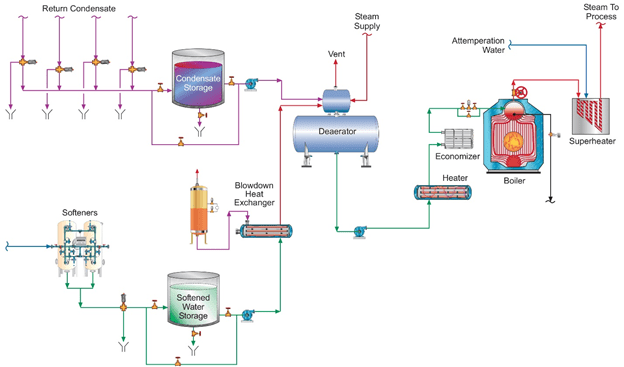
General schematic of a common industrial steam generation flow diagram. Note the multiple condensate return lines to the system.
Upon reaching the boiler, contaminants may cause internal fouling and scaling of boiler tubes, or they may influence boiler water chemistry and induce carryover of impurities to steam. One dramatic example from a number of years ago at an organic chemicals plant came from transport of organic products to four package boilers, which caused severe foaming and carryover in each drum. Fouling of the superheater tubes, and subsequent tube overheating, required superheater replacement every 1.5–2 years.
Regarding condensate return chemistry monitoring, a standard parameter, similar to all other liquid streams, is pH. Typically, the optimum pH range for these systems is 9–10, with some variation depending on the various metallurgies. If copper alloys are present, pH at the lower end of the range is usually best. Measuring pH in high-purity water can be problematic and subject to error, but at the mildly basic range of 9–10, accurate values can be calculated from specific conductivity and cation conductivity (also known as conductivity after cation exchange [CACE]) measurements. A summary of this technique is outlined in a January 2021 article in Power Engineering.
Additional condensate return instrumentation can be selected based on the primary impurities that could appear. For the chemical plant mentioned above, total organic carbon (TOC) analyses would have been a logical choice. TOC is also a strong consideration at refineries, petrochemical plants, natural gas liquefaction plants, and so forth.
At facilities that manufacture inorganic chemicals, a number of analyses have potential value, including sodium and the afore-mentioned CACE, which is actually a surrogate measurement for chloride and sulfate in high-purity water and steam. On-line hardness monitoring may fit some applications to minimize transport of calcium and magnesium to boilers, which may then precipitate as hard deposits. Referring to Part 1 of this series, hardness monitoring of sodium softener effluent can alert operators to makeup system upsets before serious issues arise.
Iron (and if necessary, copper) analyses are useful for evaluating corrosion protection, or lack thereof, in condensate return systems. A problem that plagues many steam generators is transport of iron oxide corrosion products to the boilers, where the materials precipitate, generally on the hot side of the boiler tubes. These deposits then act as locations for under-deposit corrosion (UDC), in which acid or caustic will concentrate at the tube surface and directly attack the tube metal. UDC can be more severe in high-pressure boilers with high heat fluxes, but low-pressure boilers are often operated for years and years without any chemical cleaning, such that deposits become very thick and induce UDC.
Contact ChemTreat today to request a consultation regarding your steam generation systems. Our experienced team can help you design a monitoring and treatment program to suit the unique needs of your facility.
The last post in this blog series focused on monitoring water and steam chemistry in lower-pressure boilers, with specific focus on condensate return.
This installment examines recommended monitoring parameters for the boilers in a steam generating system. Factors that influence boiler water treatment include boiler design, boiler/steam pressure, whether the steam is utilized strictly for process heating or for driving turbines, and issues related to condensate return and impurity ingress to the boiler water.
Power plant steam generators generally approach closed loop operation, because the steam only drives a turbine and is then returned in its entirety to the boiler. For the normally small amounts of water/steam that are lost, makeup comes from high-purity treatment systems, which normally ensures clean boiler water.
So, the primary monitoring parameter for utility boiler water is pH. The recommended range may vary slightly depending on the boiler type and operating pressure, but, in general, most guidelines call for a range somewhere between 9.2 and 9.8.
For the numerous units with water-cooled steam condensers, impurity ingress is always a possibility that may cause severe upsets in the high-temperature boiler environment. In these cases, pH becomes the most critical measurement. Virtually all industry guidelines recommend taking a unit off-line immediately if the pH drops to 8.0.
Tri-sodium phosphate (Na3PO4, TSP) in small dosages is still a common way to (temporarily) protect against contaminant ingress until the unit can be shut down. In some cases, small concentrations of caustic (NaOH) may be preferable over TSP.
Note: In the most common type of heat recovery steam generator (HRSG), the feed forward low-pressure (FFLP) design, solid alkalis cannot be utilized for low-pressure evaporator treatment. Rather, pH is controlled via condensate/feedwater ammonia treatment.
For most high-pressure applications, the following analyses are recommended.
The conductivity measurements, especially CACE combined with phosphate readings, can be valuable to ensure chemistry is balanced and minimizes corrosion potential. When collected and analyzed by a software program such as ChemTreat’s CTVista®+, this data can provide accurate analyses and trending of boiler water chemistry.
For industrial boilers, additional monitoring may be necessary. In many cases, makeup water treatment may only consist of sodium softening or softening and alkalinity removal. Furthermore, numerous industrial plants have complex steam and condensate return systems that may allow extra impurity ingress to the boilers. Often, the primary impurities are iron oxide corrosion products from the condensate return piping.
A common boiler water treatment program for these systems includes phosphate and a polymer, the latter to keep particulates in solution.
If hardness ingress is not an issue, polymer-only programs may be preferable. In either case, determining polymer concentrations can be a challenging task. One method is to blend a trace amount of a fluorescent compound in with the product formulation. The very small quantity of the tracer compound does not interfere with chemical reactions but can be readily analyzed to provide a surrogate measurement of the chemical concentration.
Another possibility, which first gained traction in the cooling water industry a number of years ago, involves tagged polymers. A non-reactive molecule is added to the primary polymers to make them detectable by instrumentation. In cases where tagged polymers are applicable, monitoring is done directly and not by surrogate methods.
Additional details regarding power boiler sampling may be obtained from the “Technical Guidance Document – 2015 Revision: Instrumentation for monitoring and control of cycle chemistry for the steam-water circuits of fossil-fired and combined cycle power plants”; the International Association for the Properties of Water and Steam (www.iapws.org).
The previous installments outlined monitoring criteria to protect steam generator condensate/feedwater systems and boilers from corrosion and deposition. Steam chemistry monitoring is equally important, particularly at power plants and co-generation facilities where the steam drives turbines.
Contaminant deposition on turbine blades can lead to corrosion and possible blade failures, which represent a potentially catastrophic situation with the turbine spinning at several thousand rpm.
Primary monitoring parameters include the following:
Sodium provides a direct indication of salt or sodium hydroxide carryover into steam. Sodium hydroxide (caustic) carryover is a particularly harmful impurity, as it can quickly induce stress corrosion cracking (SCC) of turbine components. Other salts, most notably sodium chloride, will settle in the last rows of the low-pressure (LP) turbine, where they can induce pitting and subsequent SCC and corrosion fatigue (CF) of turbine blades and rotors. Pitting is initiated during unit shutdowns, particularly if humid ambient air enters the condenser and moistens the deposits.
CACE provides a surrogate measurement of chloride and sulfate concentrations, and the ≤0.2 µS/cm limit has been a long-time guideline for turbine manufacturers. However, the accuracy of CACE is suspect, as the steam could have chloride and sulfate levels higher than typical 2 ppb limits, even while CACE stays below the 0.2 µS/cm parameter. Some instrumentation can analyze both impurities at well below 1 ppb concentration.
Silica will precipitate on turbine blades. Though the compound is not corrosive, deposits can influence turbine aerodynamics and reduce efficiency, hence the recommended limit noted above.
Several steam sampling points are common in power generating units. These include saturated, main, and reheat steam samples.
The saturated steam sample provides analyses of impurities coming directly from the boiler drum and can serve as a troubleshooting measurement to detect mechanical carryover. Mechanical carryover can be caused by damage or failure of a steam separating device in the drum.
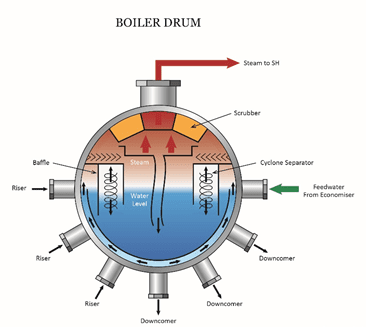
Damage or wear of separating devices allows excessive moisture containing boiler water impurities to enter the steam.
Other factors that can influence mechanical carryover include rapid firing rates or frequent load changes that cause surges in drum water level; inadequate drum size; and boiler water contamination that may generate foam.
Special procedures are required to collect saturated steam to ensure sample integrity. This includes installing an isokinetic sampling nozzle. Major sample panel manufacturers can provide information on the proper methods to ensure integrity of all steam system samples.
Main and reheat steam analyses provide direct data on impurities entering the turbine. Of course, impurity ingress may be coming from drum mechanical carryover, but impurities can also come from contaminated steam attemperation water. Such contamination should also appear in the feedwater samples and thus be detectable at that point. Detection and correction of any mechanism that contaminates feedwater is of great importance, both to protect boiler water and eliminate direct introduction of impurities to steam.
To learn more and request a consultation of your steam generation systems, contact ChemTreat. Our experienced team can help you design a monitoring and treatment programs suited to your plant’s unique needs.
Please contact ChemTreat for assistance in designing a treatment program customized for your application. Like all other technologies, due diligence is necessary to determine the feasibility for utilizing methods. Always consult your equipment manuals and guides.
