-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
By Brad Buecker, Senior Technical Publicist and Tim Hughes, Senior Technical Staff Consultant
This article was originally published in PPCHEM® Journal; PPCHEM® 2020, 22(6), 252–259; https://journal.ppchem.com/
High-pressure steam generators for power production require high-purity makeup and feedwater and controlled boiler water chemistry to minimize corrosion and scale formation in the boilers, superheater/reheater circuits, and turbines. Numerous articles in the PPCHEM® journal over the last two decades have outlined these chemistries and their evolution.
However, while many heavy industries have high-pressure steam generators for co-generation needs, these plants and many other smaller facilities also have low-pressure boilers that produce process steam. The lower heat fluxes and pressures in these steam generators somewhat alleviate the stringent treatment requirements necessary for high-pressure units but offer more complexity in the choice of optimum treatment methods.
This article provides an overview of modern methods for protecting lower-pressure steam generators from factors that typically do not plague their high-pressure counterparts.
High-pressure steam generators for power production require high-purity makeup and feedwater and controlled boiler water chemistry to minimize corrosion and scale formation in the boilers, superheater/reheater circuits, and turbines. However, while many heavy industries have high-pressure steam generators for co-generation needs, these plants and many other smaller facilities also have low-pressure boilers (boilers of less than 4.14MPa (600psig)) that produce process steam. The lower heat fluxes and pressures in these steam generators somewhat alleviate the stringent treatment requirements necessary for high-pressure units but offer more complexity in the choice of optimum treatment methods. Potential issues such as contaminated condensate return and makeup water treatment system malfunctions can increase the complexity of steam generation water/steam treatment.
This article provides an overview of modern methods for protecting lower-pressure steam generators from factors that typically do not plague their high-pressure counterparts.
In utility systems, the steam is condensed and returned to the boiler after performing its work in the turbine. The complete water/steam circuit is nearly a closed loop with approximately 0.5–2% water loss and corresponding makeup additions. Mature technologies such as reverse osmosis (RO) and ion exchange are available to produce high-purity makeup (≤2μg∙L–1 of sodium and chloride, ≤10μg∙L–1 of silica, and ≤0.1μS∙cm–1 specific conductivity). In the absence of a condenser tube leak or, less frequently, makeup system upset, the feedwater remains highly pure on its path to and through the steam generator and turbine, and for that small portion utilized for steam attemperation.
Now consider the realm of low-pressure steam generation, where the boilers do not require demineralized makeup. For decades, and even today, sodium zeolite softening has been a common primary treatment method for industrial boiler makeup. In this process, the makeup passes through a bed of ion exchange resin that trades calcium and magnesium hardness ions for sodium. The softened stream, with the remaining impurities including bicarbonate alkalinity (HCO3–), chloride (Cl–), sulfate (SO42–), silica (SiO2), and others, then feeds the boiler. Some softening makeup systems include a splitstream de-alkalizer, or perhaps a forced-draft decarbonator, to remove most of the alkalinity. This can be beneficial, as will be outlined.
Basic softening offers both advantages and drawbacks. Compared to complete demineralization, softening saves plants money in equipment and operating costs. Regenerating softening resins with brine is straightforward and does not require storing and handling dangerous acids and caustic. The major difficulty with softening is that the ions not removed by the process can become problematic upon reaching the steam generator. Alkalinity is a prime example. If it is not removed from the makeup, alkalinity will at least partially convert to carbon dioxide (CO2) in the boiler, carrying over with the steam. Upon steam condensation, the CO2 can lower the pH, leading to potential condensate corrosion issues for carbon steel piping.
The introduction of other water impurities to the boiler can lead to higher conductivity, increasing the general corrosion potential of the water, especially because the ions "cycle up" in drum boilers as steam is produced. While some accumulation of these impurities is tolerable, in many cases, plant personnel do not track deposit buildup in boilers, particularly of iron oxide corrosion products transported from elsewhere, e.g., condensate return systems. Boiler water impurities can concentrate under these deposits to much higher levels than in the bulk water, and induce under-deposit corrosion.
Heavy deposits also restrict heat transfer, and in areas of high heat flux may lead to tube overheating and mechanical failure as shown in Figure 1, where iron oxides from condensate return created thick deposit layers followed by fishmouth opening of the tube from overheating [1].
RO technology offers a reliable option for producing makeup water with very low dissolved solids. At many power plants, RO serves as the primary demineralization step with mixed-bed ion exchange or continuous electrodeionization (CEDI) polishing as the final stage, but RO as a stand-alone process can suffice for many low-pressure boilers. The process removes the bulk of impurities (often 99% or more), including silica, which can allow for higher boiler water cycles of concentration, thus saving costs via reduced makeup and blowdown.

Sufficient pretreatment to remove suspended solids ahead of RO membranes and a well-designed chemical treatment program to minimize scale formation are important to successful RO unit operation. Careful RO feedwater analysis is critical for proper pretreatment equipment and chemical selection. Also, RO generates a nearsteady wastewater stream that must be disposed of. For plants with cooling towers, the tower basin may serve as a good repository. Otherwise, alternative disposal methods may be needed.
There are many examples of makeup system upsets wherein plant personnel operated the systems in failed mode or sometimes even bypassed malfunctioning systems and fed raw water to the boiler. A mindset of "water is water" seems to prevail in these cases. Such assumptions can lead to disastrous consequences, and boiler tubes have been known to fail within days or sometimes hours of such decisions.
Apart from whatever method is utilized for makeup water production, significant impurities can enter the steam generator via condensate return from plant processes. The percent condensate return may range from slight to very large depending on plant design and operation. In a classic example of contaminated condensate observed years ago by one of the authors, superheater bundle replacement of four package steam boilers at an organic chemicals plant was required every 1.5–2 years because of internal deposition and overheating failures. The root cause was excessive organic ingress to the condensate return, which induced foaming in the boiler drums and solids carryover to the superheaters. No systems were in place either to polish the condensate or to dump it during impurity excursions.
Depending on the chemical processes at the plant and the ability of impurities to enter the condensate, a wide variety of contaminants can potentially enter the boiler. A program should be in place to detect chemical leakage from heat exchangers, reactors, or other vessels, and to make repairs as needed. Testing condensate return for pH, hardness, and specific conductivity is common. And, it might be prudent to check the return condensate for organics in specific cases. With such monitoring, the condensate can be diverted to drain if the measurement exceeds a predetermined limit, e.g., 50μS∙cm–1 continuous on-line conductivity. Setpoints for dump or reuse of return condensate should be defined for all site-specific parameters that might impact boiler feedwater quality. Condensate dumping can be expensive considering the costs for makeup water production and heating water to produce steam; however, plant personnel may rely too much on the boiler water treatment program to alleviate problems. Excessive contamination can overload any treatment program.
As with high-pressure steam generators, establishing and maintaining a moderately basic pH range (in general, pH 9–10) is an important issue for feedwater and condensate return systems in low-pressure units to prevent general carbon steel corrosion. In the power industry, the common pH conditioner is ammonia, which raises feedwater pH via the following reaction:

This is a reversible reaction, so the alkalinity increase is limited, which usually minimizes excessive steel corrosion in the event of a chemical feed upset. (High ammonia concentrations, especially in the presence of oxygen, can be very detrimental to copper alloys.) Conditions are often different in low-pressure boilers. If the makeup water is sodium-softened only, sufficient alkalinity may be present to maintain a basic pH. Sometimes a bit of caustic feed may be employed to boost feedwater pH, although care must be taken when using this strong chemical.
However, the wild card for industrial systems is condensate return, in which the pH may be significantly depressed by carbon dioxide carryover. Accordingly, neutralizing amine injection to condensate return is often employed to minimize corrosion in carbon steel piping networks.
A common injection point is the storage section of the deaerator or directly to the steam header, which may be better. The chemical or chemical blend not only protects the condensate but carries through the system. Table 1 details several of the most common neutralizing amines.
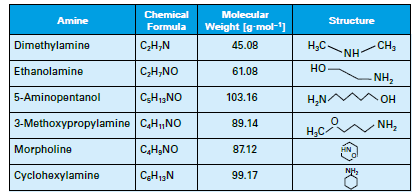
Table 1: List of common neutralizing amines.
The amines all have a higher molecular weight than ammonia, and thus will not flash off as readily, although each has its own distribution ratio (the amount remaining in the water vs. that departing with steam) whose properties are a function of temperature and pressure. The products also have different basicities, providing flexibility in treatment program selection. Careful evaluation of condensate return system operating and design conditions is necessary to select the most appropriate amine or amine blend.
Some compounds are not allowed if the steam can directly contact food or other consumable products.
As discussed in a previous issue of the PPCHEM® journal, monitoring total iron concentrations in condensate is highly recommended to evaluate feedwater chemistry efficacy [2]. We will return to this idea again later in this article.
In the middle of the last century, the consensus about dissolved oxygen (DO) in boiler feedwater was uniform: oxygen should be eliminated because it is highly corrosive. But European and Russian researchers in the late 60s and early 70s discovered that some dissolved oxygen (at concentrations up to 300μg·L–1) introduced to high-purity water during normal operation induced formation of a tight α-hematite oxide layer on carbon steel piping. Corresponding particulate and dissolved feedwater iron concentrations could be driven to very low values of 1μg·L–1 or even less. The program became known as oxygenated treatment (OT) and was widely applied to once-through supercritical units in Europe and, eventually, in the United States and elsewhere. The caveat for OT is that it requires exceptionally high-purity feedwater (≤0.15μg·L–1 cation conductivity); otherwise, oxygen corrosion may occur. Figure 2 illustrates this concept.
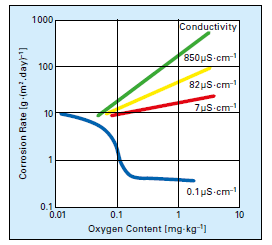
Figure 2: Oxygen corrosion rates as a function of dissolved solids content. [3]
Drum units continued to operate with feedwater chemistry anchored to dissolved oxygen removal by mechanical deaeration and reducing agent (also known as oxygen scavenger) treatment until failures from flow-accelerated corrosion (FAC) began to emerge in the 1980s. A number of these failures caused injuries and fatalities at power plants over the next three decades. FAC chemistry has been well documented in the PPCHEM® journal [4] and other publications, but a key point is that it led to the development of a cousin to OT for drum units named all-volatile treatment, under oxidizing conditions (AVT(O)), which also relies on a small concentration of dissolved oxygen in the feedwater. AVT(O) also requires high-purity feedwater (≤0.2μg·L–1 cation conductivity) to develop the proper oxide layer on carbon steel while minimizing oxygen corrosion.
The upshot of this brief discussion about OT and AVT(O) is that industrial boilers are usually supplied with less than high-purity makeup, and often recover condensate that contains some impurities, so neither AVT(O) nor OT can be utilized because of the potential for severe oxygen corrosion of carbon steel components. Most industrial feedwater systems are equipped with mechanical deaerators, which, when operating properly, should reduce the DO concentration to 7–10μg·L–1. A common layout for industrial low-pressure steam generation is displayed in Figure 3.
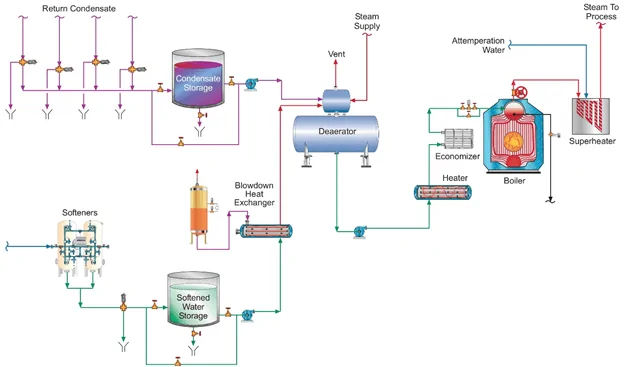
A chemical reducing agent is also typically employed to further lower DO levels. For steam generators at or below a pressure of 4.14MPa (600psi), either un-catalyzed or catalyzed sodium sulfite (Na2SO3) is a popular reducing agent. This non-volatile oxygen scavenger adds some inorganic dissolved solids to the feedwater.
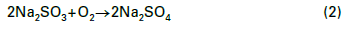
A common injection point is the deaerator storage tank.
But as has already been observed many times in the power industry, complete DO removal leads to conditions that promote FAC. This raises an important question, "Can FAC occur in industrial feedwater systems if the DO concentration is reduced to very low values?" The answer is yes, and reference [5] outlines mild cases of FAC at a co-generation facility in the United States. To summarize, recent non-destructive testing revealed some wall loss at elbows on both the suction and discharge side of several boiler feedwater pumps as well as at a nozzle and a weld seam. None require immediate repair, but they prompted plant personnel to plan additional testing to ensure other locations are not seriously damaged. Undoubtedly, a mitigating factor in the mild nature of those spots suffering from FAC is that plant personnel have strived to maintain feedwater pH within a mid- to upper-9 range, in accordance with data presented by Sturla [6] nearly five decades ago (Figure 4).
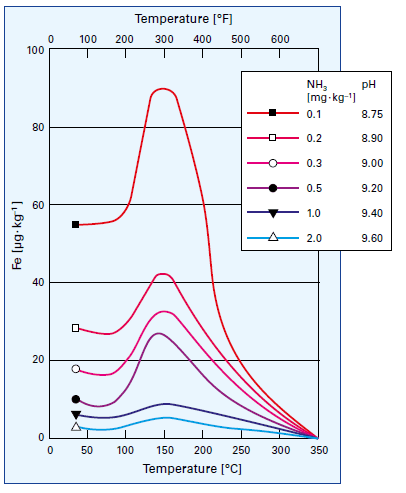
Figure 4: Influence of temperature and pH on iron dissolution from carbon steel [5]. The temperature aspect is why FAC is typically most pronounced in the feedwater systems and economizers of many conventional steam generators and the LP evaporators of multi-pressure heat recovery steam generators (HRSGs).
An important point to note is that this elevated pH range would be too high for most systems containing copper alloys and would need to be lowered to the low 9s for combined iron and copper corrosion control. It is in such situations that analytical techniques like corrosion product sampling can be quite valuable, where the solution concentration of both metals can be evaluated. For all-ferrous systems, straight iron monitoring techniques are possible, and reference [1] outlines several of these techniques. The International Association for the Properties of Water and Steam (IAPWS) has generated a Technical Guidance Document (TGD) [7], which discusses the variety of analytical methods that can be used for these tests. At the facility highlighted in reference [5], personnel make spot checks of condensate and feedwater iron concentrations via the well-known Millipore test method, in which a known volume of the sample is passed through a very small-pore (0.45μm) white filter paper, whose color is compared to standard samples after drying. The Millipore procedure was in large measure pioneered and promoted years ago by the boiler manufacturer Babcock & Wilcox for quick calculations of feedwater iron concentrations during unit startups [8]. In utility boilers, virtually all of the particulates will be iron oxides, but in industrial steam generators with complex steam and condensate return networks, other impurities that mask the results may exist.
In many complex industrial steam generation/condensate return systems, it is desirable to have proper pH control throughout the network, but a single compound may not be sufficient to achieve this control. The authors' colleagues have developed blended amine products that can provide wide-ranging coverage. A thorough analysis of system design, metallurgy, current chemistry, and operating temperatures is a prerequisite for proper program selection.
Film-forming amines (FFA) were introduced by the water treatment industry decades ago, and recent years have seen a re-emergence of film-forming substances (especially octadecylamine or C18H39N) for corrosion protection. The amine group on each molecule attaches to the metal substrate, and the long-chain organic portion of the molecule extends into the water and acts as a barrier. However, poor control and lack of detailed knowledge of the overall chemistry have often led to problems, including formation of "gunk balls" that fouled steam generators [9]. Advancements in chemical synthesis techniques and analytical instrumentation have led to the development of new film-forming substances, both amines and other compounds, that are much more effective at protecting metal surfaces. This includes the FFA products of ChemTreat's TITAN360™ series. Figure 5 shows a protected metal surface (during off-line conditions). Note how the water beads rather than wetting the surface.
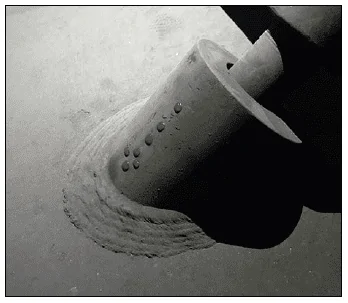
Figure 5: Protected metal surface with FFA.
When correctly applied, film-formers can protect metal surfaces during normal operation and unit downtimes. However, it must be noted that implementing FFA programs requires proper system oversight and control. Less-than-knowledgeable vendors have been known to suggest that such chemicals could be injected into the unit, after which corrosion issues would magically disappear. Severe problems were the result instead. A careful analysis of system operation and past/present chemistry is necessary beforehand, with careful monitoring and control required after FFA chemistry is introduced, as it is explicitly described and recommended in section 8 of the IAPWS TGD11-19 [9].
In the 1930s, as power generating units increased in number and size, tri-sodium phosphate (Na3PO4, also known as TSP) became a popular boiler water conditioning chemical for drum boilers. At that time, phosphate treatment served two primary functions. The first was to establish moderately alkaline conditions in the boiler to minimize general corrosion of carbon steel boiler tubes, drums, and headers.
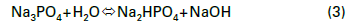
This function is still quite critical today.
A second function of phosphate was, and in many cases still is for industrial boilers, important for scale control where hardness ingress occurs. Eq. (4) below outlines the most common of these scale-forming reactions, which has probably been observed ever since humans began heating water for personal and then industrial use.
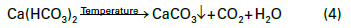
Calcium carbonate (CaCO3) deposition, often accompanied by other minerals, still plagues many industrial steam generators when makeup systems malfunction but remain in operation or are bypassed.
Phosphate and the alkalinity produced by its reaction with water will react with hardness ions to form soft sludges as opposed to hard scale. However, in the early days of power unit operation, some boilers were plagued by under-deposit caustic corrosion generated by the rather high concentrations of TSP needed for scale control. This led to the development of coordinated and congruent phosphate treatment programs that utilized blends of tri-, di-, and sometimes even a small amount of monosodium phosphate. Subsequent research has shown that these chemistries can generate acidic phosphate deposits in high-pressure steam generators. Utility boiler treatment programs have returned to TSP (or, in some cases, caustic treatment), albeit in low dosages of perhaps no greater than 2mg·L–1. This is possible because modern makeup treatment systems are quite reliable, such that hardness in-leakage is very rare. Thus, phosphate treatment is used for pH control only.
For industrial boilers, phosphate treatment remains a strong choice, particularly because the potential for hardness ingress to many industrial units is much greater. The lower heat fluxes in these steam generators allow higher phosphate dosages than in utility units. And it may be possible to sometimes employ phosphate blends rather than TSP alone for more flexibility in pH control. Sludge conditioners consisting of water-soluble polymers that help keep solids in suspension by a combination of dispersion, crystal modification, and sequestration are often recommended alongside phosphate treatment. Iron particulates from condensate return system corrosion can be problematic, but sludge conditioners help to keep the particles in suspension for subsequent blowdown. These polymers can sometimes serve as a standalone treatment, particularly if hardness ingress is not an issue.
In former days, chelants were sometimes employed in industrial drum units. These chemicals directly bind with metals to keep them suspended. Ethylenediaminetetraacetic acid (EDTA) is the most widely known chelant and has been used for many applications both inside and outside industrial applications. However, improper chelant use can cause localized corrosion of boiler components. Chelant programs are very rare now and should only be used with well-deaerated feedwater, excellent pretreatment control, and low feedwater iron concentrations.
The upshot is that several possibilities, namely phosphate/polymers, polymers alone, and, rarely, chelating agents, exist for boiler water treatment, but the proper choice depends on a variety of factors that include boiler design and pressure, makeup water treatment sophistication and reliability, and the potential for impurity ingress and iron oxide carryover from condensate return. These factors must be evaluated carefully for each case. A "one size fits all" approach to treatment selection can lead to problems.
The lower pressures and heat fluxes in industrial steam generators make them less susceptible to high-temperature corrosion mechanisms than those in utility units. However, chemical treatment of these lower-pressure units may be more complicated because of several factors, including:
Careful planning is necessary to establish the proper treatment programs for the entire steam generation system and condensate return networks. A variety of methods are available to optimize chemistry. Comprehensive monitoring is necessary to ensure treatment programs are performing as intended.
The authors would like to greatly thank Frank Udo Leidich of the PPCHEM® journal International Advisory Board for reviewing this article and providing valuable information.

Tim Hughes (B.S., Petroleum and Natural Gas Engineering, Pennsylvania State University, State College, PA, USA) is a senior technical staff consultant with ChemTreat. He has 29 years of industrial water treatment experience and 8 years of oil & gas experience. He previously held positions at Betz Laboratories, Universal Well Services and National Fuel Gas Supply Corp.
