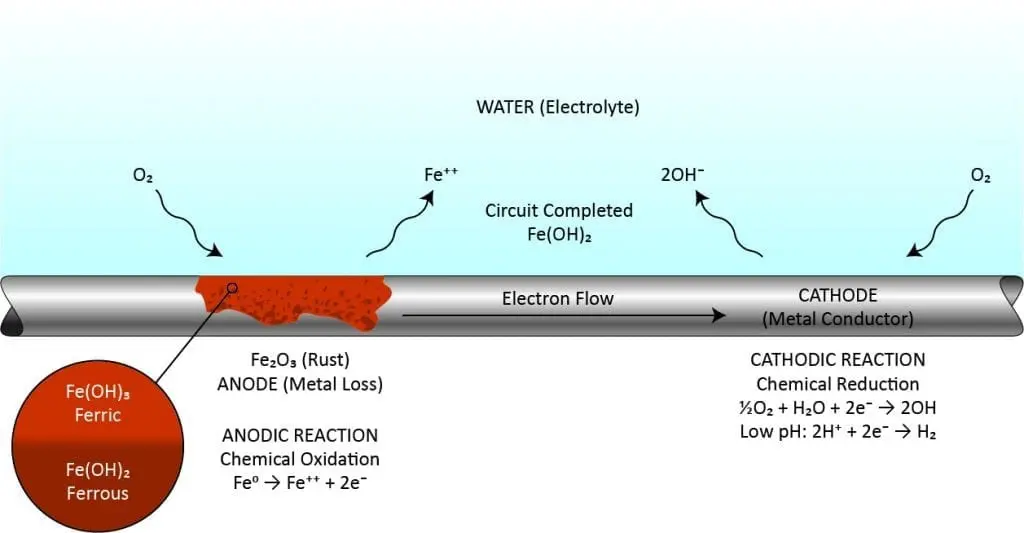
These chemistries are mainly used in closed loop cooling systems for corrosion inhibition. Molybdate (MoO4-2) combines with iron ions formed at anodes to produce a molecular ferric-molybdate film at the metal surface. If molybdate residuals are properly maintained, the film will eventually cover the entire metal surface. Research also shows that molybdates acts as a pitting inhibitor with its ability to accumulate within the acidic part of a pit and block that corrosion process. Nitrites (NO2–), which are generally restricted to closed cooling water treatment, actually passivate metal and cause it to form its own protective layer. Silicates react with metal ions at the anode to form a protective film. Sodium tetraborate pentahydrate (Na2B4O7•5H2O) is an anodic inhibitor that protects ferrous metals from corrosion. It is thought that borate produces a ferric-borate layer on metal surfaces, encouraging the formation of ferric oxide, which acts as a barrier to ion transport, particularly ferric ions, away from the metal surface. A relatively weak inhibitor, it is typically used along with other inhibitors like nitrite or silicates. A key feature of borates (metaborate) is they provide good buffering capacity for pH slightly above 9.0.
-
USA - English
- Locations
- SDS Access
- CTVista®+ Login