-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
Pharmaceutical companies (as well as semi-conductor manufacturers and high-pressure steam generating power stations) require high-purity makeup water for their processes. Impurity concentrations have to be in the very low parts-per-billion (ppb) range or even lower. Reliable and efficient production of high-purity water is integral to these efforts.
In the last century, the development of synthetic ion exchange resins was a major steppingstone for high-purity water production. Resins to remove dissolved cations such as calcium, magnesium, sodium, etc. and anions, chloride, sulfate, and even weakly ionized silica became a workhorse for treatment systems. However, it became apparent that, when utilized as the primary purifying technique for makeup water, ion exchange resins can quickly become exhausted because of the substantial concentration of ions in the makeup supply, even from fresh water sources. These systems often required daily regenerations of exchange resins with strong acid for cation resins and caustic for anion resins.
The development of membrane technologies significantly altered this process. A common core configuration for high-purity makeup water preparation in modern industry is outlined below.
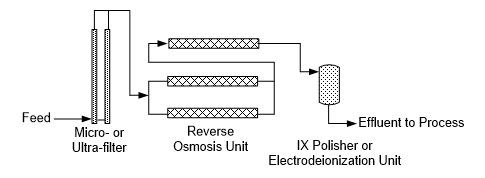
Modern reverse osmosis (RO) membranes can remove over 99% of dissolved solids, which requires polishing the RO permeate by ion exchange or electrodeionization (EDI), or sometimes both in series, to produce high-purity makeup.
Pretreatment is a critical aspect of reliable RO unit operation, particularly minimizing fine particulate carryover to RO membranes. Many 20th-century water treatment systems were designed with clarifiers, followed by multi-media filters, to remove most of the suspended solids from the incoming plant makeup stream. Like other technologies, clarification has been greatly improved over time, and modern clarifiers may now operate at 10–20 times greater rise rates than the conventional large, circular clarifiers of the past. However, fine particulates can still escape with the clarifier/filter effluent and foul RO membranes, especially the leading elements. Microfiltration (MF) or ultrafiltration (UF) is often recommended for new makeup water systems to provide low suspended solids RO feed. MF or UF may be placed downstream of a clarifier or may sometimes serve as stand-alone filtration devices.
Unlike RO elements, which utilize spiral-wound membranes (as will be shown in Part 2 of this series), microfilter and ultrafilter membranes typically have the hollow-fiber configuration, in which the membranes resemble long spaghetti strands or fibers, typically called lumens. Pressure-type systems are common.
In this type of MF unit, the fibers are housed within pressure vessels connected in parallel.


The unit shown in Figure 2 was chosen as a replacement for an aging clarifier, whose primary function was to remove suspended solids from a lake water supply. Results from the changeover were immediate, and in some cases readily observable. For example, the turbidity of the inlet stream to the RO unit dropped from a typical range of 0.5–1.0 NTU (nephelometric turbidity units) to less than 0.05 NTU. The time between RO cartridge filter changeouts increased from weeks to months. In addition, upstream MF or UF improves RO performance, typically reduces the frequency of membrane cleanings, and extends membrane life.
Ultrafiltration is very common today, offering even finer filtration than MF. Table 1 outlines general pore sizes for the four major membrane technologies.
Table 1 – General Membrane Pore Sizes
| Rule of Thumb Membrane Pore Sizes |
| Microfiltration – 0.1 µm |
| Ultrafiltration – 0.01 µm |
| Nanofiltration – 0.001 µm |
| Reverse osmosis – 0.0001 µm |
An important point to remember is that MF and UF are designed solely for particulate removal, while nanofiltration and RO remove dissolved ions. The functions cannot be interchanged.
Fundamentals of Pharmaceutical Water Treatment
Pharmaceutical plants typically contain numerous batch reactors, distillation columns, crystallizers, and additional heat exchangers, which require both open evaporative and closed cooling water systems, making well-maintained cooling tower chemistry essential to plant reliability and efficiency.
The diagram below illustrates the problematic issues that can plague cooling systems, and how each issue can influence the others.
Watch the full webinar online.
Another fundamental aspect of these systems is cooling tower discharge. For nearly four decades, the most common corrosion/scale control program for industrial cooling towers relied on a core chemistry of inorganic and organic phosphate compounds. This chemistry establishes mildly alkaline conditions in cooling water to reduce the potential for general corrosion, and it relies on controlled deposition of phosphate compounds, including calcium and iron phosphate, for additional corrosion control. A small concentration of zinc is often included for additional corrosion control, with the organic phosphate (aka, phosphonate) portion and a supplemental polymer providing scale inhibition.
However, there is an increasing concern over the impact of phosphorus on the environment, particularly the growing issue of toxic algae blooms in natural bodies of water. In many locations, industrial discharge containing phosphorus is being regulated.
To mitigate this issue, ChemTreat developed our proprietary, sustainable, and non-fouling FlexPro® technology. The majority of FlexPro products do not contain phosphate. This non-phosphorus formulation interacts directly with the metal surfaces to form a protective layer, which, unlike phosphate/phosphonate programs, does not rely on deposition of reaction products to form this barrier.
Full-scale application of this chemistry has proven very effective. At one chemical plant, the two-pass heat exchanger was suffering from corrosion on the low-temperature inlet side and calcium phosphate deposition on the high-temperature outlet side from reverse solubility of phosphates at higher temperatures. FlexPro helped the customer solve both problems.

Before FlexPro Treatment

After FlexPro Treatment
For pharmaceutical plants that produce a variety of products and may have numerous cooling systems of different sizes and complex metallurgies, FlexPro may be a great treatment option. Easier to control than traditional phosphate/phosphonate chemistries, this innovative technology helps facilities reduce phosphate discharge to meet their environmental goals while maintaining superior corrosion performance without fouling concerns.
Steam is widely utilized in the pharmaceutical industry for many heat transfer and sterilization processes. Rigorous control of steam generation chemistry is also necessary to prevent corrosion and fouling of equipment that could otherwise cause failures and process shutdowns.
Boiler condensate/feedwater system treatment is a key aspect of protecting steam generation equipment. Typically, most of the piping in these networks is of carbon steel construction. Treatment programs are normally geared to minimize two primary corrosion mechanisms: general corrosion and oxygen attack. General carbon steel corrosion is often addressed by maintaining pH within a mildly alkaline range from near 9 to 10.
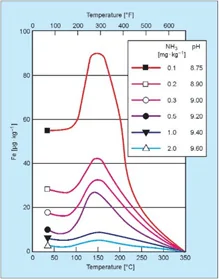
Figure 1. Influence of temperature and pH on iron dissolution from carbon steel. Source: Sturla, P., Proc., Fifth National Feedwater Conference, 1973, Prague, Czechoslovakia.
In the power industry, the common pH conditioner is ammonia, which raises feedwater pH via the following reaction:
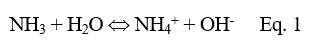
However, ammonia is typically not preferred for systems where steam can touch food or pharma products. The most common alternatives are neutralizing amines, which are short-chain organic compounds with either an attached or embedded amine group.
Even then, only some of these compounds satisfy regulatory requirements, and they often have concentration limits.
Accordingly, treatment products and injection points should be selected with care, and residual concentrations should be monitored. ChemTreat’s experienced team can work with you to develop the best pH conditioning program for your facility. We can tailor a blended amine treatment specifically to your system needs.
Most industrial steam generator systems include a mechanical deaerator (DA).
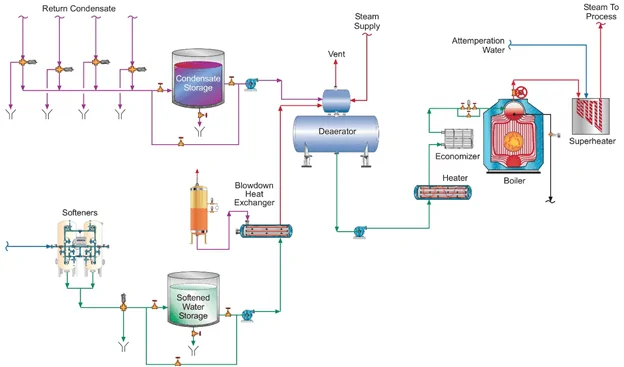
Figure 2. A common industrial steam generation layout. Note the makeup water and condensate return feeds to the deaerator.
However, the dissolved oxygen (D.O.) lower limit guaranteed by DA manufacturers is typically 7 part-per-billion (ppb) in the DA storage tank discharge. To further reduce D.O. concentrations in the boiler feedwater, many facilities feed a supplemental reducing agent, aka an oxygen scavenger.
Oxygen scavengers common to heavy industry applications, such as carbohydrazide, hydroquinone, etc. are often not suitable for pharma facilities with low-pressure boilers. Non-volatile sodium sulfite (Na2SO3) is one alternative.
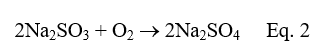
This product generally does not carry over in steam, unless poor boiler operation or malfunction of drum steam separator devices allows mechanical incursion of moisture into the steam. Another compound popular for D.O. removal in special applications is erythorbic acid.
Like sodium sulfite, it is a non-volatile reducing agent.
Contact us for assistance in designing a treatment program customized to your specific needs. Like all other technologies, due diligence is necessary to determine the feasibility for utilizing methods. Always consult your equipment manuals and guides and seek ChemTreat guidance to address your specific system needs..
