Over time, high-pressure, high-purity steam generators such as waste heat boilers will experience corrosion and deposition. Industrial water treatment experts can help chemical, petrochemical, and refining facilities minimize these effects to enhance system efficiency and reliability.
This article provides:
- 1. A basic overview of high-pressure, high-purity steam cycle chemistry for the chemical, petrochemical, and refining industries
- 2. An introduction to the four pillars of steam cycle treatment
- 3. A discussion of typical steam generator issues at chemical processing plants, as well as opportunities for improvement
- a. Anionic and cationic impurities
- b. Corrosion and condensation
- c. Lack of steam quality monitoring and instrumentation
We hope this discussion will help chemical facility personnel improve the long-term production and reliability of their steam systems.
Why Boiler Water Treatment Matters
An Overview of High-Pressure, High-Purity Steam Cycle Chemistry
The primary purpose of boiler water treatment is reducing corrosion throughout the steam cycle.
Treating boilers differs slightly from cooling tower, closed loop, and other chemical system treatment. For instance, true corrosion inhibitors are not added to a boiler system. Instead, aggressive anions like calcium, magnesium, chlorides, sulfates, and silicas are removed.
Oxygen is also removed via mechanical and chemical means.
Higher pH and temperatures stimulate the natural passivation processes that reduce corrosion and emissions, so boiler treatment typically involves raising the pH as well.
The secondary purpose of boiler water treatment is reducing deposition following pretreatment.
Iron and, to a lesser degree, copper deposition is particularly problematic, as it can transport into steam generators and boilers. Iron transported into boilers deposits in high heat flux areas, potentially leading to underdeposit corrosion.
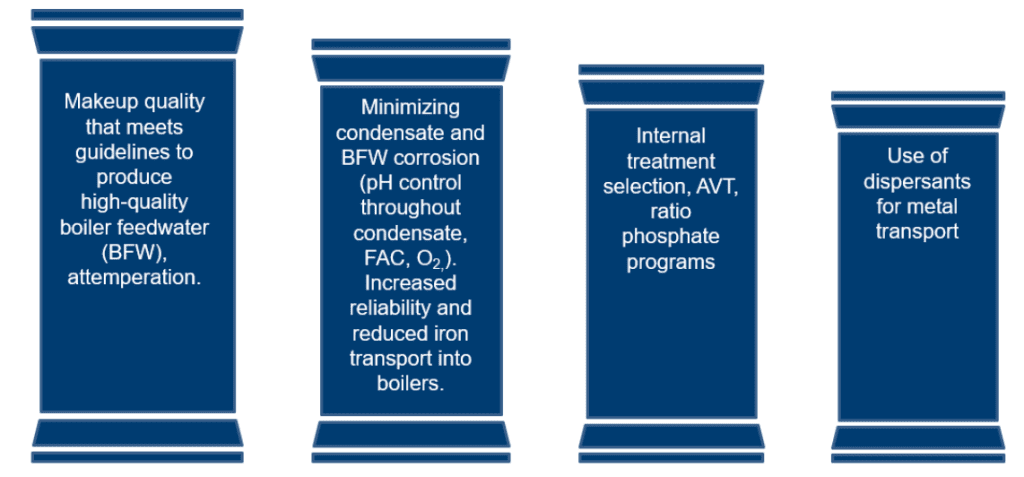
The Four Pillars of Steam Cycle Treatment
The four pillars of steam cycle treatment are a useful tool for understanding boiler treatment and the common gaps seen at chemical processing facilities.
In descending level of importance, the pillars are:
- 1. High-quality feedwater production
- 2. Condensate and corrosion reduction
- 3. Internal treatment selection and AVT
- 4. Dispersant treatment for metal transport
It is important to note that:
- If the first two pillars are addressed with minimal gaps, the second two pillars become less problematic.
- If there are gaps or failures in the first two pillars, the second two similarly matter less, as system reliability issues become more prevalent.
Pillar 1: Producing High-Quality Feedwater through High-Quality Makeup
The first pillar represents a particularly challenging issue in the chemical processing industry. High-quality feedwater is created through the attemperation of high-quality makeup, otherwise known as boiler feedwater (BFW). This process involves controlling and measuring low-level ionic impurities to avoid boiler feedwater contamination, as contaminants can find their way into the steam through the attemperation process.
Pillar 2: Minimizing Condensate and Boiler Feedwater Corrosion
The second pillar focuses on minimizing condensate and BFW corrosion. This is typically accomplished by applying amines or ammonia, as well as through oxygen scavenging.
Generally, the goal is to minimize corrosion products and iron transport through the system. More specifically, we will explore flow-accelerated corrosion (FAC), another major gap faced by chemical and petrochemical plants as well as refineries.
Pillar 3: Selecting the Appropriate Internal Treatment
The third pillar addresses the importance of selecting the right steam gyrator and boiler internal treatment based on a system’s specific needs. This could include all-volatile treatment (AVT) ratio programs using sodium phosphate or all-polymer programs that can be used at lower pressures.
Pillar 4: Using Dispersants for Metal Transport
The last pillar refers to using polymers to disperse iron. In certain instances, this treatment is recommended for reducing iron transport into steam generators and boilers.
Three Common Steam Cycle Treatment Gaps in Chemical Processing Facilities
The four pillars provide a solid foundation for a deeper discussion into the specific challenges many chemical, petrochemical, and refining facilities face.
- 1. Anionic and cationic impurities
- 2. Accelerated corrosion and condensate
- 3. Lack of steam quality monitoring and instrumentation
1. Anionic Impurities and Cationic Impurities
Feedwater quality is a major area of improvement for many chemical processors. In fact, demineralized water quality drives the majority of boiler chemistry needs.
Unfortunately, many plants do not follow best practices and operate boilers with low levels of ionic impurities in their makeup water. Controlling and measuring low-level ionic impurities is an important factor for maintaining boiler efficiency.
Cation Slipping
Plants typically use some form of mixed bed ion exchange to produce higher purity water.
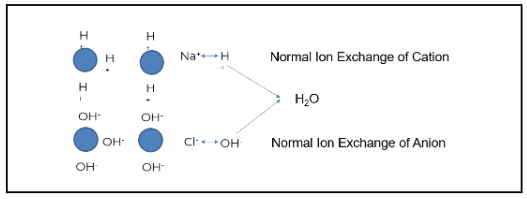
The image above demonstrates how this process works when ion exchange is properly generated and operating as intended. The blue circles represent ion beads. When regenerated, the cation is loaded with a hydrogen or hydronium ion. The anion bead is loaded with a hydroxyl ion.
Using the example of salt (NaCl) and water (H2O), sodium (the cation) exchanges with a hydrogen ion. Chloride (the anionic portion) exchanges with a hydroxyl ion, creating pure water. Under the right conditions, regeneration produces high-quality feedwater. However, gaps such as cation bed slipping can occur when the ion exchange does not operate as designed.
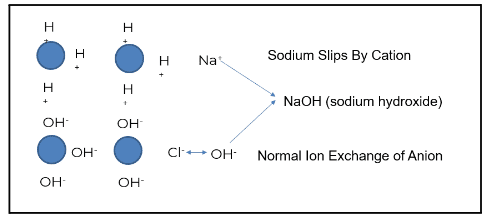
Sodium is a prevalent ion seen when the cation bed starts to slip. In the example above, sodium does not exchange with the hydronium or hydrogen ion; this is known as “slipping.” The slip causes the sodium to go through the chloride. Sodium chloride then exchanges out with a hydroxyl ion, and sodium slips as sodium hydroxide.
The sodium hydroxide will first concentrate in the boiler water, driving up boiler pH. This will increase the sodium-to-phosphate ratio, which, in turn, increases the risk of underdeposit corrosion and caustic gouging. Attemperation contamination reduces the steam quality for turbines, and calcium and magnesium can pass under more stressed conditions.
Even slightly elevated sodium levels pose a risk of sodium hydroxide formation in iron deposits. When this occurs, porous iron deposits in boilers (known as “wick boiling,” which will be discussed later in this article), the boiler water evaporates off, and high levels of iron concentrate remain.
If sodium hydroxide reaches the feedwater, which is normally used for de-superheating, it could directly contaminate the steam going to the turbine blades, reducing efficiency. Hydroxide can also deposit onto the turbine blades in the phase transition zone as it starts to condense, potentially causing stress corrosion cracking, usually on the roots, where the turbine blades attach to the rotor.
Under extreme conditions, as the cation resin is exhausted, the hardness ions of calcium and magnesium will start to pass, which can cause deposit issues in boilers at higher temperatures. Boilers are virtually intolerant to hardness at this level, and calcium magnesium can react with the alkalinity and start to drop out pH in the boiler.
Anion Slipping
Though not as common as cation slipping, anion slipping is an important point to discuss. Interestingly, slipping anions has the opposite effect of a slipping cation. The first anion we typically see slip is silica. Slipping silica combines with the exchanged hydronium ion, forming a slightly acidic species. Depending on the temperature, pressure, and pH, silica in these high-pressure, high-purity boilers is prone to mechanical and vaporous carryover in drums. This poses a risk to turbines; silica forms deposits on turbine blades, reducing efficiency.
Another problem with silica is that, unlike sodium, it cannot be removed with water washing. Silica forms a very tenacious deposit on turbine blades that is difficult to remove.
Other anions that can slip are chlorides and sulfates, which combine with the hydrogen ion to form mineral acids, such as hydrochloric and sulfuric acid, driving down boiler pH. These increase the risk of underdeposit corrosion in porous iron deposits.
If mineral acids enter the steam, they provide another mechanism for stress corrosion cracking in turbine blades.
2. Accelerated Condensate and Corrosion
When it comes to minimizing condensate and corrosion, the main priority is reducing levels of iron and other metal transport into the boilers.
Iron and Copper Transport into Steam Generators
Water systems in chemical processing facilities tend to be large, with complicated condensate systems and extensive steam cycles. Typically, the feedwater going into the boiler will have elevated iron levels, or even yellow metals. Any metal entering the boiler may deposit on boiler surfaces; it is difficult to keep them in solution in higher heat flux areas. This creates potential sites for underdeposit corrosion.
The following images show examples of underdeposit corrosion. The first is a transfer line exchanger (TLE) from a methanol plant, where waste heat gas was sent through the tubes. Acid phosphate wastage caused the formation of underdeposit corrosion sites. The plant had switched to a much higher-level sodium phosphate program years before, but the damage was already done.
The following images show examples of underdeposit corrosion. The first is a transfer line exchanger (TLE) from a methanol plant, where waste heat gas was sent through the tubes. Acid phosphate wastage caused the formation of underdeposit corrosion sites. The plant had switched to a much higher-level sodium phosphate program years before, but the damage was already done.

This represents a major challenge of underdeposit corrosion: once it occurs, bulk water chemistry will have minimal effect on existing underdeposit corrosion sites.
The second image is an example of a thick wall failure in a utility boiler. It shows an entire blowout, known as hydrogen damage. This was caused by a very acidic species depositing within the steel matrix and converting the carbon sites over to methane, leading the wall to expand and eventually resulting in the large failure seen here.
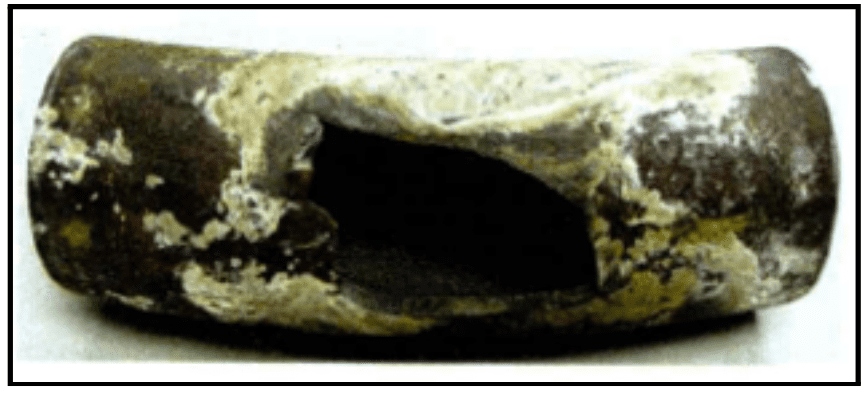
Image courtesy of Kurt Kraetsch, ChemTreat
These images illustrate the importance of pretreatment to boiler efficiency. Effective pretreatment reduces iron and other metal transport into the preboiler. Approximately 30% of iron entering a boiler remains soluble and is taken out via continuous blowdown.
Polymer treatment can help reduce metal transport and may be a good option for boilers with a history of underdeposit corrosion issues.
Wick Boiling
The following graphic demonstrates a principle called wick boiling, where porous iron deposits form on a boiler tube’s higher heat flux areas. These deposits occur when water with ionic impurities flows into the tube and evaporates off, creating high levels of concentration of these acidic and caustic species. This results in underdeposit corrosion in the form of tube wastage. Wick boiling typically causes hydrogen damage at a pH of 4, acid phosphate at low pH, and caustic gouging at high pH.
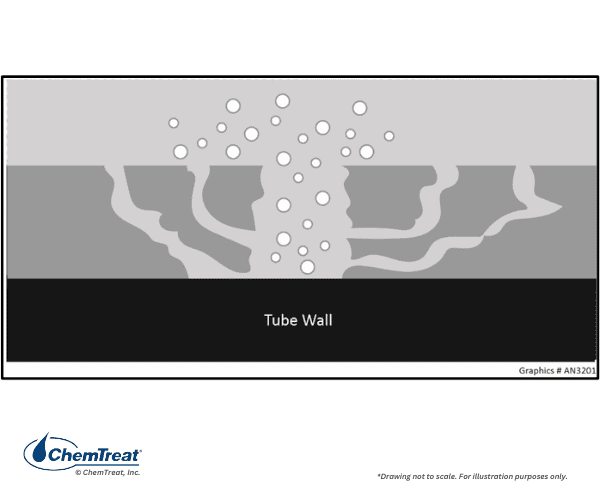
Balancing Mild Steel and Copper Corrosion in the Preboiler
Mixed-metallurgy boilers pose additional water treatment challenges. These boilers are typically made with mild steel and some form of yellow metal, such as copper and copper alloys like nickel and brass.
Copper and steel require completely different types of treatment to inhibit corrosion, which makes it difficult to calibrate treatment accordingly.
For copper, corrosion inhibition usually involves lowering pH to the 7–8 range. Higher levels of oxygen scavenger are typically added to accomplish this.
Mild steel, on the other hand, responds better to amines and a higher pH.
Balancing pH and amine/ammonia and oxygen scavenger feed is a constant challenge in mixed metallurgy systems.
Flow-Accelerated Corrosion: Feedwater and Condensate
Controlling flow-accelerated corrosion (FAC) presents one of the most significant gaps in water treatment for the chemical industry. FAC occurs when the rate of oxygen, or the rate at which the magnetite layer dissolves, is greater than its rate of formation.
Though this concept has long been well-understood, best practices for its mitigation has not been consistently applied in the chemical industry.
The following graphic illustrates the FAC cycle.
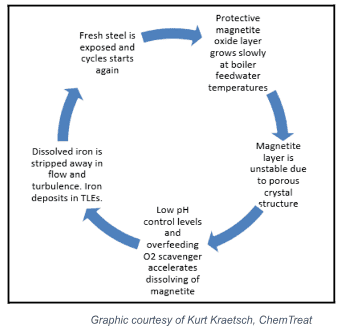
The process begins in the upper right-hand corner. The oxide layer grows slowly at first, as boiler feedwater temperatures are mild.
The magnetite layer continues to grow, developing an unstable, porous crystal structure.
Controlling ammonia/amine and oxygen scavenger comes into play at the next stage, as lower pH and high oxygen scavenger levels accelerate the dissolution of the magnetite layer.
As the iron is dissolved, it is stripped away in high-flow and turbulent areas, forming deposits in TLEs and boilers.
This leaves the fresh metal exposed, restarting the cycle.
Reducing FAC Potential with Feedwater pH Control
The graphic below shows the solubility of the magnetite layer, representing the risk of FAC.
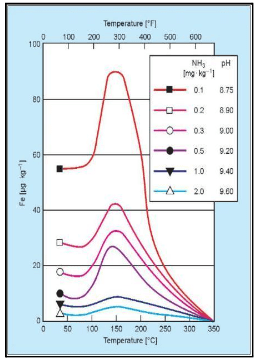
The horizontal axis displays the temperature, while the vertical axis correlates to solubility. The risk peaks at approximately 300°F, or 150°C, which roughly corresponds to standard feed system operation, depending on deaerator pressure.
The individual-colored lines represent pH. As pH increases, the system’s ability to resist the dissolution of the magnetite layer increases. The highest solubility shown on this graph is a pH 8.75, dropping off significantly as pH increases to the 9.4–9.6 range. Thus, balancing feedwater and condensate operation at the highest practical pH, while effectively treating yellow metals, can be very difficult.
Understanding the Impact of Oxygen on FAC
The impact of oxygen on FAC is not well understood. Oxygen works synergistically with aggressive anions like chlorides and sulfates, causing corrosion. However, oxygen can also help reduce FAC.
As previously mentioned, the magnetite layer forming in feedwater systems is porous. If the gaps in the layer are filled with hematite by slightly increasing oxygen ingress, a stronger oxide layer will develop, which is less likely to dissolve and cause FAC.
In all-steel systems, pH is not always set as high as necessary, and reducing or removing oxygen scavenger application may help reduce FAC, depending on iron transport analysis.
3. Lack of Steam Quality Monitoring and Instrumentation
As with the improvement opportunities discussed in the previous section, following best practices around monitoring and instrumentation in steam systems is an important factor of effective water treatment.
Monitoring steam quality helps maintain the purity of the steam going to the turbines. Some common monitoring parameters are reviewed in the next sections, followed by a discussion of the benefits of using instrumentation over relying solely on sampling.
Sodium and Silica
Sodium and silica deposits, as well as aggressive anions like chlorides and sulfates, can cause stress corrosion cracking at the wet ends of turbine blades, reducing system efficiency.


These analytical reports show the presence of various impurities in a 600-pound ethylene unit during standard operations versus a condenser leak. It is important to note the conductivity showed very little change during the condenser leak. This facility used ultra-low-level wet-based testing to track impurities but did not have sodium monitoring in place. Using instrumentation to monitor sodium may have caught the condenser leak earlier.
Monitoring feedwater and steam with instrumentation is a water treatment best practice. However, these systems may be difficult and expensive to retrofit and maintain.
One alternative is monitoring cation conductivity, which provides a good option for testing low-level ionic impurities.
Cation Conductivity Monitoring
There are two primary types of cation conductivity monitoring.
- 1. Degassed cation conductivity, where the water is boiled off, then cooled back down and run through a strong cation exchange column, typically to remove CO2. Newer units may use a nitrogen purge to remove CO2.
- 2. Un-degassed cation conductivity monitoring, in which water is run through the cation exchange column without being boiled off.
Photo courtesy of Hach.
Regardless of whether water is degassed, the key is to measure the conductivity before and after the cation exchange column.
Two Principles of Cation Conductivity Monitoring
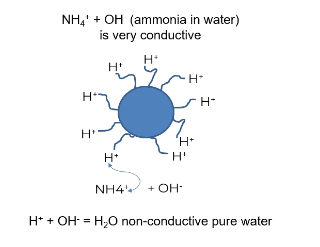
- 1. The majority of conductivity found in steam, feedwater, and condensate systems comes from adding ammonia or amines, not from ionic impurities. Monitoring cation conductivity removes the impact of ammonia or amines on the conductivity reading. The diagram below shows ammonia as ammonia hydroxide in water. The ammonium portion (the cation) will exchange out with a hydronium or hydroxyzine to form pure water, taking out the masking effect produced by amines or ammonia.
- 2. Cation conductivity converts low levels of impurities to acids, which are always more conductive than their neutral salts. During a condenser leak or a period of poor ion exchange, sodium chloride is a typical impurity. The sodium in sodium chloride exchanges out with a hydronium hydroxyl or hydrogen ion to form hydrochloric acid, amplifying the conductivity’s effect by taking low part per billion levels of ionic impurities and converting them into acids.
- a. During the ethylene unit condenser leak shown previously, there was a slight uptake in normal, un-neutralized, or non-cation, conductivity. The average conductivity increased from 3–4.5 to 3.5–4.5. It would typically be difficult to note this difference; however, the cation conductivity shot up by a factor of 10In areas such as feedwater, steam, and condensate systems, cation conductivity offers an efficient way to measure and monitor very low levels of ionic impurities via instrumentation.
The Benefits of Instrumentation Over Sampling
The example above illustrates the importance of instrumentation in helping facilities catch boiler system issues.
Some chemical processing plants still rely solely on “grab” samples, which provide an incomplete picture of the system water quality. Particularly in high-pressure, high-purity units, monitoring with instrumentation is best practice, with grab sampling used only as a backup.
Conclusion
Minimizing corrosion and deposition in high-purity, high-pressure steam generators and boilers is an important component of maintaining system reliability and efficiency in chemical, petrochemical, and refining facilities.
When designing a water treatment program, it is important to keep the four pillars of steam water treatment in mind, as well as identifying areas of improvement based on common gaps.
The ultimate goal of this post was to address these gaps in high-purity, high-pressure steam generator treatment and help your facility improve the long-term reliability of your boilers and steam turbines by implementing best practices around treatment chemistries and monitoring.
As with all other technologies, due diligence is necessary to determine the feasibility of utilizing the methods discussed in this post. It is important to consult your equipment manuals and guides and seek guidance from your local water treatment representative to address the specific needs of your facility.


